| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] |
|---|
| Ligand | BDBM203873 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | TR-FRET Binding A |
|---|
| pH | 7.4±n/a |
|---|
| Temperature | 297.15±n/a K |
|---|
| Ki | 44±0 nM |
|---|
| IC50 | 162±23 nM |
|---|
| Comments | extracted |
|---|
| Citation |  Akçay, G; Belmonte, MA; Aquila, B; Chuaqui, C; Hird, AW; Lamb, ML; Rawlins, PB; Su, N; Tentarelli, S; Grimster, NP; Su, Q Inhibition of Mcl-1 through covalent modification of a noncatalytic lysine side chain. Nat Chem Biol12:931-936 (2016) [PubMed] Article Akçay, G; Belmonte, MA; Aquila, B; Chuaqui, C; Hird, AW; Lamb, ML; Rawlins, PB; Su, N; Tentarelli, S; Grimster, NP; Su, Q Inhibition of Mcl-1 through covalent modification of a noncatalytic lysine side chain. Nat Chem Biol12:931-936 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] |
|---|
| Name: | Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] |
|---|
| Synonyms: | BCL2L3 | Induced myeloid leukemia cell differentiation protein Mcl-1(171-327) | MCL1 | MCL1_HUMAN | Myeloid cell leukemia 1 (Mcl-1) |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 17896.13 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q07820[171-327] |
|---|
| Residue: | 157 |
|---|
| Sequence: | EDELYRQSLEIISRYLREQATGAKDTKPMGRSGATSRKALETLRRVGDGVQRNHETAFQG
MLRKLDIKNEDDVKSLSRVMIHVFSDGVTNWGRIVTLISFGAFVAKHLKTINQESCIEPL
AESITDVLVRTKRDWLVKQRGWDGFVEFFHVEDLEGG
|
|
|
|---|
| BDBM203873 |
|---|
| n/a |
|---|
| Name | BDBM203873 |
|---|
| Synonyms: | 7-(3-((4-Boronophenoxy)methyl)-1,5-dimethyl-1H-pyrazol-4-yl)-3-(3-(naphthalen-1-yloxy)propyl)-1H-indole-2-carboxylic acid, 9 | Mcl-1 inhibitor 9 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C34H38BN3O6 |
|---|
| Mol. Mass. | 595.493 |
|---|
| SMILES | CC1C(C(COc2ccc(cc2)B(O)O)NN1C)c1cccc2C(CCCOc3cccc4ccccc34)C(Nc12)C(O)=O |
|---|
| Structure | 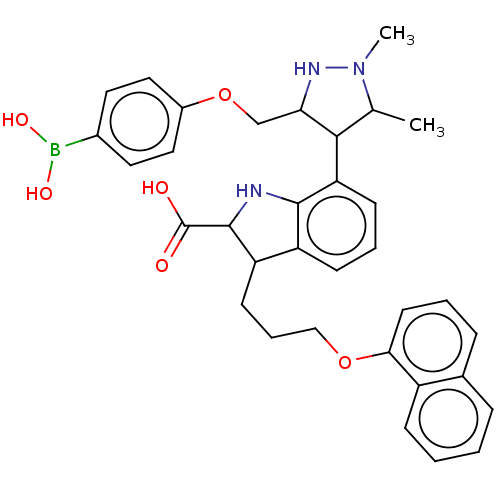
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Akçay, G; Belmonte, MA; Aquila, B; Chuaqui, C; Hird, AW; Lamb, ML; Rawlins, PB; Su, N; Tentarelli, S; Grimster, NP; Su, Q Inhibition of Mcl-1 through covalent modification of a noncatalytic lysine side chain. Nat Chem Biol12:931-936 (2016) [PubMed] Article
Akçay, G; Belmonte, MA; Aquila, B; Chuaqui, C; Hird, AW; Lamb, ML; Rawlins, PB; Su, N; Tentarelli, S; Grimster, NP; Su, Q Inhibition of Mcl-1 through covalent modification of a noncatalytic lysine side chain. Nat Chem Biol12:931-936 (2016) [PubMed] Article