| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prostaglandin G/H synthase 2 [18-604,S516A] |
|---|
| Ligand | BDBM50312668 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | COX Enzyme Inhibition Assay (Table 2) |
|---|
| IC50 | 1.5e+3±n/a nM |
|---|
| Citation |  Uddin, MJ; Crews, BC; Xu, S; Ghebreselasie, K; Daniel, CK; Kingsley, PJ; Banerjee, S; Marnett, LJ Antitumor Activity of Cytotoxic Cyclooxygenase-2 Inhibitors. ACS Chem Biol11:3052-3060 (2016) [PubMed] Article Uddin, MJ; Crews, BC; Xu, S; Ghebreselasie, K; Daniel, CK; Kingsley, PJ; Banerjee, S; Marnett, LJ Antitumor Activity of Cytotoxic Cyclooxygenase-2 Inhibitors. ACS Chem Biol11:3052-3060 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prostaglandin G/H synthase 2 [18-604,S516A] |
|---|
| Name: | Prostaglandin G/H synthase 2 [18-604,S516A] |
|---|
| Synonyms: | Cox-2 | Cox2 | Cyclooxygenase-2 mutant (S530A) | PGH2_MOUSE | Pghs-b | Ptgs2 | Tis10 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 67244.20 |
|---|
| Organism: | Mus musculus (Mouse) |
|---|
| Description: | Sequence based on 4OTJ |
|---|
| Residue: | 587 |
|---|
| Sequence: | ANPCCSNPCQNRGECMSTGFDQYKCDCTRTGFYGENCTTPEFLTRIKLLLKPTPNTVHYI
LTHFKGVWNIVNNIPFLRSLIMKYVLTSRSYLIDSPPTYNVHYGYKSWEAFSNLSYYTRA
LPPVADDCPTPMGVKGNKELPDSKEVLEKVLLRREFIPDPQGSNMMFAFFAQHFTHQFFK
TDHKRGPGFTRGLGHGVDLNHIYGETLDRQHKLRLFKDGKLKYQVIGGEVYPPTVKDTQV
EMIYPPHIPENLQFAVGQEVFGLVPGLMMYATIWLREHNRVCDILKQEHPEWGDEQLFQT
SRLILIGETIKIVIEDYVQHLSGYHFKLKFDPELLFNQQFQYQNRIASEFNTLYHWHPLL
PDTFNIEDQEYSFKQFLYNNSILLEHGLTQFVESFTRQIAGRVAGGRNVPIAVQAVAKAS
IDQSREMKYQSLNEYRKRFSLKPYTSFEELTGEKEMAAELKALYSDIDVMELYPALLVEK
PRPDAIFGETMVELGAPFALKGLMGNPICSPQYWKPSTFGGEVGFKIINTASIQSLICNN
VKGCPFTSFNVQDPQPTKTATINASASHSRLDDINPTVLIKRRSTEL
|
|
|
|---|
| BDBM50312668 |
|---|
| n/a |
|---|
| Name | BDBM50312668 |
|---|
| Synonyms: | CHEMBL1076638 | Chemocoxib A (12) | N-{(Succinylpodophyllotoxinyl)but-4-yl}-2-{1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl}acetamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C49H50ClN3O13 |
|---|
| Mol. Mass. | 924.387 |
|---|
| SMILES | COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NCCCCNC(=O)CCC(=O)O[C@H]3[C@@H]4COC(=O)[C@H]4[C@@H](c4cc(OC)c(OC)c(OC)c4)c4cc5OCOc5cc34)c2c1 |r| |
|---|
| Structure | 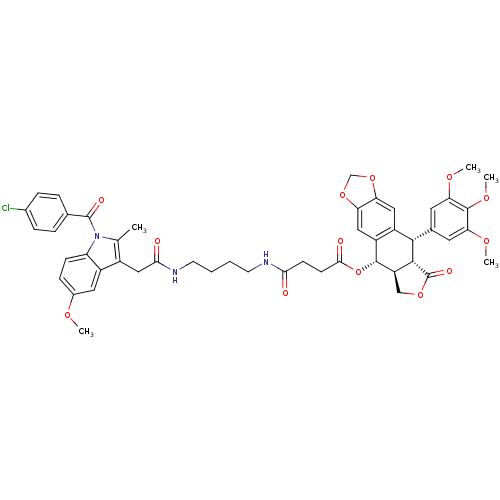
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Uddin, MJ; Crews, BC; Xu, S; Ghebreselasie, K; Daniel, CK; Kingsley, PJ; Banerjee, S; Marnett, LJ Antitumor Activity of Cytotoxic Cyclooxygenase-2 Inhibitors. ACS Chem Biol11:3052-3060 (2016) [PubMed] Article
Uddin, MJ; Crews, BC; Xu, S; Ghebreselasie, K; Daniel, CK; Kingsley, PJ; Banerjee, S; Marnett, LJ Antitumor Activity of Cytotoxic Cyclooxygenase-2 Inhibitors. ACS Chem Biol11:3052-3060 (2016) [PubMed] Article