

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Muscarinic acetylcholine receptor M3 | ||
| Ligand | BDBM221901 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Muscarinic Receptor Binding Assay | ||
| IC50 | 2.2±n/a nM | ||
| Citation |  Prat Quinones, M; Fonquerna Pou, S; Puig Duran, C; Lumeras Amador, W; Aiguade Bosch, J; Caturla Jovaloyes, JF Cyclohexylamine derivatives having β2 adrenergic agonist and M3 muscarinic antagonist activities US Patent US9315463 Publication Date 4/19/2016 Prat Quinones, M; Fonquerna Pou, S; Puig Duran, C; Lumeras Amador, W; Aiguade Bosch, J; Caturla Jovaloyes, JF Cyclohexylamine derivatives having β2 adrenergic agonist and M3 muscarinic antagonist activities US Patent US9315463 Publication Date 4/19/2016 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Muscarinic acetylcholine receptor M3 | |||
| Name: | Muscarinic acetylcholine receptor M3 | ||
| Synonyms: | ACM3_HUMAN | CHRM3 | Cholinergic, muscarinic M3 | Muscarinic Receptors M3 | Muscarinic receptor M3 | RecName: Full=Muscarinic acetylcholine receptor M3 | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 66151.03 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P20309 | ||
| Residue: | 590 | ||
| Sequence: |
| ||
| BDBM221901 | |||
| n/a | |||
| Name | BDBM221901 | ||
| Synonyms: | US9315463, 10 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C38H41ClN4O7S2 | ||
| Mol. Mass. | 765.338 | ||
| SMILES | CN(CCC(=O)Nc1ccc(CNC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1Cl)[C@H]1CC[C@@H](CC1)OC(=O)C(O)(c1cccs1)c1cccs1 |r,wU:14.14,34.40,wD:31.33,(4.15,5.39,;4.15,3.85,;2.82,3.08,;1.48,3.85,;.15,3.08,;.15,1.54,;-1.18,3.85,;-2.52,3.08,;-2.52,1.54,;-3.85,.77,;-5.19,1.54,;-6.52,.77,;-7.85,1.54,;-9.19,.77,;-10.52,1.54,;-10.52,3.08,;-11.85,.77,;-13.19,1.54,;-14.52,.77,;-14.52,-.77,;-15.86,-1.54,;-13.19,-1.54,;-13.19,-3.08,;-11.85,-3.85,;-11.85,-5.39,;-10.52,-3.08,;-10.52,-1.54,;-11.85,-.77,;-5.19,3.08,;-3.85,3.85,;-3.85,5.39,;5.48,3.08,;5.48,1.54,;6.82,.77,;8.15,1.54,;8.15,3.08,;6.82,3.85,;9.48,.77,;10.82,1.54,;10.82,3.08,;12.15,.77,;13.49,,;12.15,-.77,;13.4,-1.68,;12.92,-3.14,;11.38,-3.14,;10.91,-1.68,;13.49,1.54,;14.95,1.06,;15.86,2.31,;14.95,3.56,;13.49,3.08,)| | ||
| Structure | 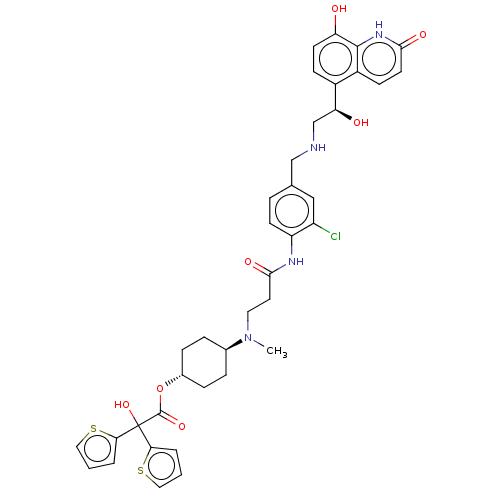
| ||