| Reaction Details |
|---|
 | Report a problem with these data |
| Target | UDP-glucuronosyltransferase 2B7 |
|---|
| Ligand | BDBM21361 |
|---|
| Substrate/Competitor | Diclofenac |
|---|
| Meas. Tech. | UDP-glucuronosyltransferase Activity Assay |
|---|
| IC50 | >3.00e+5±n/a nM |
|---|
| Citation |  Liu, Y; She, M; Wu, Z; Dai, R The inhibition study of human UDP-glucuronosyltransferases with cytochrome P450 selective substrates and inhibitors. J Enzyme Inhib Med Chem26:386-93 (2011) [PubMed] Article Liu, Y; She, M; Wu, Z; Dai, R The inhibition study of human UDP-glucuronosyltransferases with cytochrome P450 selective substrates and inhibitors. J Enzyme Inhib Med Chem26:386-93 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| UDP-glucuronosyltransferase 2B7 |
|---|
| Name: | UDP-glucuronosyltransferase 2B7 |
|---|
| Synonyms: | 2.4.1.17 | 3,4-catechol estrogen-specific UDPGT | UD2B7_HUMAN | UDP-glucuronosyltransferase 2B7 | UDP-glucuronosyltransferase 2B9 | UDPGT 2B7 | UDPGT 2B9 | UDPGTh-2 | UGT2B7 | UGTB2B9 | Uridine-5'-diphosphoglucuronosyltransferase 2B7 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 60705.98 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P16662 |
|---|
| Residue: | 529 |
|---|
| Sequence: | MSVKWTSVILLIQLSFCFSSGNCGKVLVWAAEYSHWMNIKTILDELIQRGHEVTVLASSA
SILFDPNNSSALKIEIYPTSLTKTELENFIMQQIKRWSDLPKDTFWLYFSQVQEIMSIFG
DITRKFCKDVVSNKKFMKKVQESRFDVIFADAIFPCSELLAELFNIPFVYSLSFSPGYTF
EKHSGGFIFPPSYVPVVMSELTDQMTFMERVKNMIYVLYFDFWFEIFDMKKWDQFYSEVL
GRPTTLSETMGKADVWLIRNSWNFQFPYPLLPNVDFVGGLHCKPAKPLPKEMEDFVQSSG
ENGVVVFSLGSMVSNMTEERANVIASALAQIPQKVLWRFDGNKPDTLGLNTRLYKWIPQN
DLLGHPKTRAFITHGGANGIYEAIYHGIPMVGIPLFADQPDNIAHMKARGAAVRVDFNTM
SSTDLLNALKRVINDPSYKENVMKLSRIQHDQPVKPLDRAVFWIEFVMRHKGAKHLRVAA
HDLTWFQYHSLDVIGFLLVCVATVIFIVTKCCLFCFWKFARKAKKGKND
|
|
|
|---|
| BDBM21361 |
|---|
| Diclofenac |
|---|
| Name | BDBM21361 |
|---|
| Synonyms: | (5S)-5-Ethyl-3-methyl-5-phenyl-2,4-imidazolidinedione | (5S)-5-ethyl-3-methyl-5-phenylimidazolidine-2,4-dione | Mephenytoin, D- | S-Mephenytoin | S-mephentoin |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C12H14N2O2 |
|---|
| Mol. Mass. | 218.2518 |
|---|
| SMILES | CC[C@]1(NC(=O)N(C)C1=O)c1ccccc1 |
|---|
| Structure | 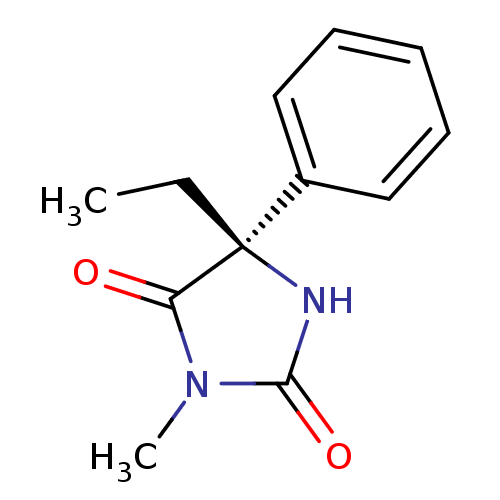
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Liu, Y; She, M; Wu, Z; Dai, R The inhibition study of human UDP-glucuronosyltransferases with cytochrome P450 selective substrates and inhibitors. J Enzyme Inhib Med Chem26:386-93 (2011) [PubMed] Article
Liu, Y; She, M; Wu, Z; Dai, R The inhibition study of human UDP-glucuronosyltransferases with cytochrome P450 selective substrates and inhibitors. J Enzyme Inhib Med Chem26:386-93 (2011) [PubMed] Article