

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Histamine receptor H3 | ||
| Ligand | BDBM247453 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Radioligand Binding Assay | ||
| Ki | 6.10±n/a nM | ||
| Citation |  Gao, Z Substituted phenyl cycloalkyl pyrrolidine (piperidine) spirolactams and amides, preparation and therapeutic use thereof US Patent US9453023 Publication Date 9/27/2016 Gao, Z Substituted phenyl cycloalkyl pyrrolidine (piperidine) spirolactams and amides, preparation and therapeutic use thereof US Patent US9453023 Publication Date 9/27/2016 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Histamine receptor H3 | |||
| Name: | Histamine receptor H3 | ||
| Synonyms: | n/a | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 48611.84 | ||
| Organism: | Macaca mulatta (Rhesus macaque) | ||
| Description: | Q865E1 | ||
| Residue: | 445 | ||
| Sequence: |
| ||
| BDBM247453 | |||
| n/a | |||
| Name | BDBM247453 | ||
| Synonyms: | US9453023, 12 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C25H36N2O2 | ||
| Mol. Mass. | 396.5655 | ||
| SMILES | C[C@H]1CCCN1[C@H]1CC[C@@H](CC1)c1ccc(cc1)N1CCC2(CCOCC2)C1=O |r,wU:9.13,wD:6.6,1.0,(6.5,-3.36,;7.27,-2.02,;8.74,-1.55,;8.74,-.01,;7.27,.47,;6.37,-.78,;4.83,-.78,;4.06,.56,;2.52,.56,;1.75,-.78,;2.52,-2.11,;4.06,-2.11,;.21,-.78,;-.56,-2.11,;-2.1,-2.11,;-2.87,-.78,;-2.1,.56,;-.56,.56,;-4.41,-.78,;-5.66,-1.68,;-6.9,-.78,;-6.43,.69,;-5.66,2.02,;-6.43,3.36,;-7.97,3.36,;-8.74,2.02,;-7.97,.69,;-4.89,.69,;-4.12,2.02,)| | ||
| Structure | 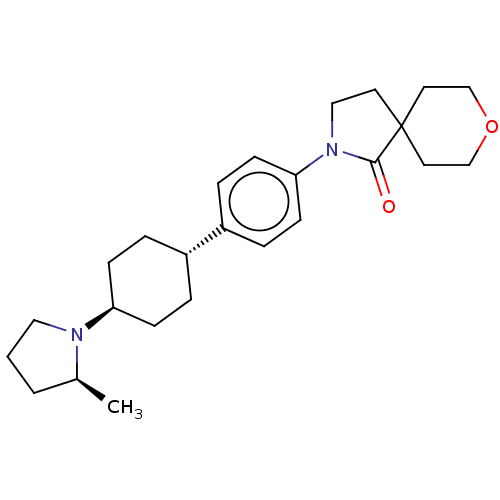
| ||