

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Integrase | ||
| Ligand | BDBM294718 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Anti-HIV Activity MT4 Assay | ||
| IC50 | 3.00±n/a nM | ||
| Citation |  Johns, BA; Velthuisen, EJ; Weatherhead, JG; Suwandi, L; Temelkoff, D Isoindoline derivatives for use in the treatment of a viral infection US Patent US10112899 Publication Date 10/30/2018 Johns, BA; Velthuisen, EJ; Weatherhead, JG; Suwandi, L; Temelkoff, D Isoindoline derivatives for use in the treatment of a viral infection US Patent US10112899 Publication Date 10/30/2018 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Integrase | |||
| Name: | Integrase | ||
| Synonyms: | Human immunodeficiency virus type 1 integrase | ||
| Type: | PROTEIN | ||
| Mol. Mass.: | 32231.48 | ||
| Organism: | Human immunodeficiency virus 1 | ||
| Description: | ChEMBL_90865 | ||
| Residue: | 288 | ||
| Sequence: |
| ||
| BDBM294718 | |||
| n/a | |||
| Name | BDBM294718 | ||
| Synonyms: | US10112899, Example 195 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C34H37F2NO6 | ||
| Mol. Mass. | 593.6575 | ||
| SMILES | COc1c(F)cccc1C(=O)N1Cc2c(C1)c(C)c(-c1cc(F)c3OCCCc3c1C)c([C@H](OC(C)(C)C)C(O)=O)c2C |r,wD:32.36,(-3.98,-8.51,;-3.21,-7.17,;-3.98,-5.84,;-5.52,-5.84,;-6.29,-7.17,;-6.29,-4.51,;-5.52,-3.17,;-3.98,-3.17,;-3.21,-4.51,;-1.67,-4.51,;-.9,-5.84,;-.9,-3.17,;.63,-3.01,;.95,-1.5,;-.38,-.73,;-1.52,-1.76,;-.38,.81,;-1.71,1.58,;.95,1.58,;.95,3.12,;2.29,3.89,;2.29,5.43,;3.62,6.2,;.95,6.2,;.95,7.74,;-.38,8.51,;-1.71,7.74,;-1.71,6.2,;-.38,5.43,;-.38,3.89,;-1.71,3.12,;2.29,.81,;3.62,1.58,;3.62,3.12,;4.95,3.89,;4.95,5.43,;6.29,3.12,;6.29,4.66,;4.95,.81,;6.29,1.58,;4.95,-.73,;2.29,-.73,;3.62,-1.5,)| | ||
| Structure | 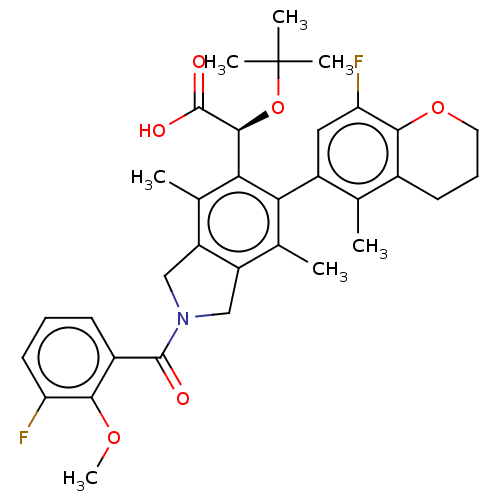
| ||