 Found 493 hits of affinity data for UniProtKB/TrEMBL: O00750
Found 493 hits of affinity data for UniProtKB/TrEMBL: O00750 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444320
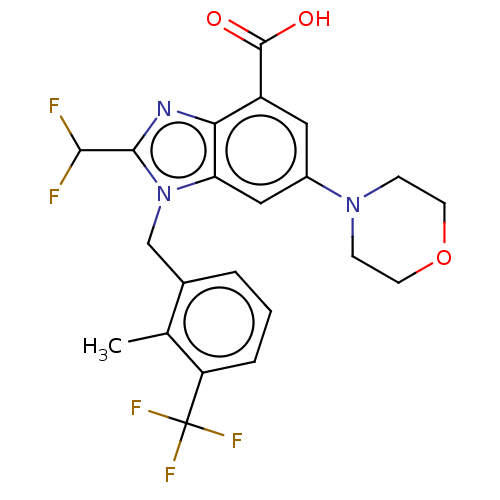
(US10660898, Example 70)Show SMILES Cc1c(Cn2c(nc3c(cc(cc23)N2CCOCC2)C(O)=O)C(F)F)cccc1C(F)(F)F Show InChI InChI=1S/C22H20F5N3O3/c1-12-13(3-2-4-16(12)22(25,26)27)11-30-17-10-14(29-5-7-33-8-6-29)9-15(21(31)32)18(17)28-20(30)19(23)24/h2-4,9-10,19H,5-8,11H2,1H3,(H,31,32) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444306

(US10660898, Example 25)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc2ccccc12)N1CCOCC1)-c1nnc[nH]1 Show InChI InChI=1S/C25H24N6O/c1-17-28-24-22(25-26-16-27-29-25)13-20(30-9-11-32-12-10-30)14-23(24)31(17)15-19-7-4-6-18-5-2-3-8-21(18)19/h2-8,13-14,16H,9-12,15H2,1H3,(H,26,27,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444315

(US10660898, Example 53)Show SMILES Cc1c(Cl)cccc1Cn1c(nc2c(cc(cc12)N1CCOCC1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C21H19ClF3N3O3/c1-12-13(3-2-4-16(12)22)11-28-17-10-14(27-5-7-31-8-6-27)9-15(19(29)30)18(17)26-20(28)21(23,24)25/h2-4,9-10H,5-8,11H2,1H3,(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444311

(US10660898, Example 39)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1)-c1nnc[nH]1 Show InChI InChI=1S/C23H23F3N6O/c1-14-16(4-3-5-19(14)23(24,25)26)12-32-15(2)29-21-18(22-27-13-28-30-22)10-17(11-20(21)32)31-6-8-33-9-7-31/h3-5,10-11,13H,6-9,12H2,1-2H3,(H,27,28,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50579668
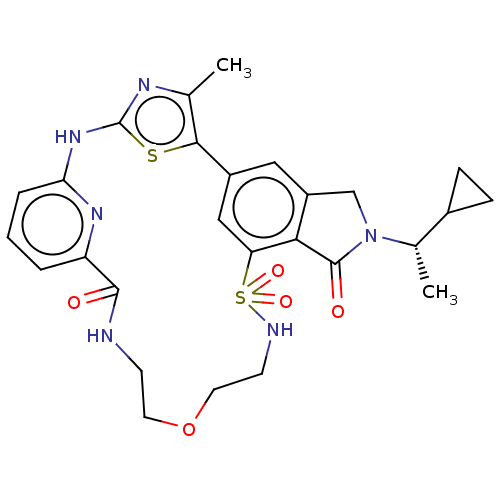
(CHEMBL5091438)Show SMILES C[C@@H](C1CC1)N1Cc2cc-3cc(c2C1=O)S(=O)(=O)NCCOCCNC(=O)c1cccc(Nc2nc(C)c-3s2)n1 |r| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIK3C2B (unknown origin) assessed as reduction in substrate phosphorylation by FRET Adapta assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM489258
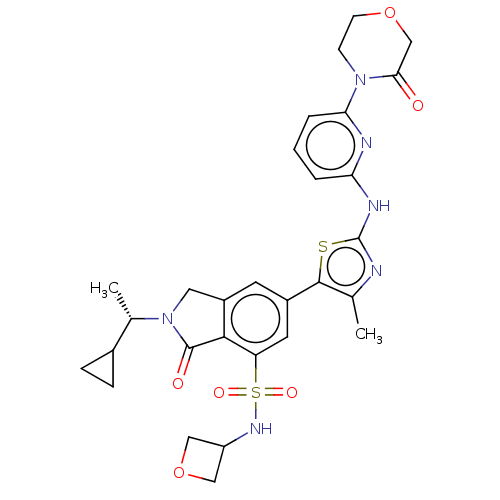
(2-[(1S)-1-Cyclopropylethyl]-6-(4-methyl-2-{[6-(3-o...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)NC1COC1)-c1sc(Nc2cccc(n2)N2CCOCC2=O)nc1C |r| Show InChI InChI=1S/C29H32N6O6S2/c1-16-27(42-29(30-16)32-23-4-3-5-24(31-23)34-8-9-40-15-25(34)36)19-10-20-12-35(17(2)18-6-7-18)28(37)26(20)22(11-19)43(38,39)33-21-13-41-14-21/h3-5,10-11,17-18,21,33H,6-9,12-15H2,1-2H3,(H,30,31,32)/t17-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIK3C2B (unknown origin) assessed as reduction in substrate phosphorylation by FRET Adapta assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444319
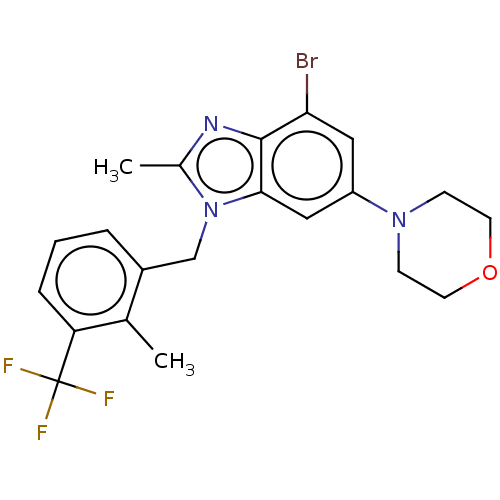
(US10660898, Example 63)Show SMILES Cc1nc2c(Br)cc(cc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C21H21BrF3N3O/c1-13-15(4-3-5-17(13)21(23,24)25)12-28-14(2)26-20-18(22)10-16(11-19(20)28)27-6-8-29-9-7-27/h3-5,10-11H,6-9,12H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444301
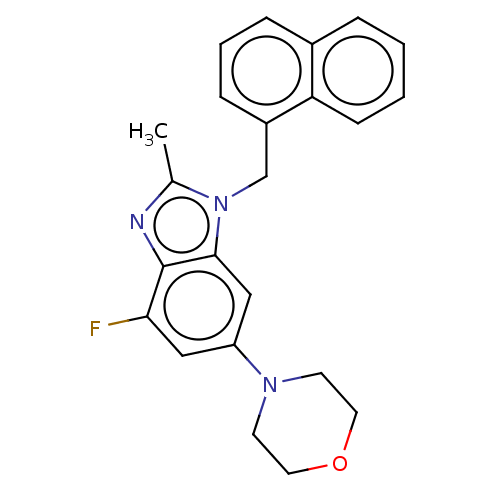
(US10660898, Example 15)Show InChI InChI=1S/C23H22FN3O/c1-16-25-23-21(24)13-19(26-9-11-28-12-10-26)14-22(23)27(16)15-18-7-4-6-17-5-2-3-8-20(17)18/h2-8,13-14H,9-12,15H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444314

(US10660898, Example 50)Show SMILES OC(=O)c1cc(cc2n(Cc3cccc4ccccc34)c(nc12)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C24H20F3N3O3/c25-24(26,27)23-28-21-19(22(31)32)12-17(29-8-10-33-11-9-29)13-20(21)30(23)14-16-6-3-5-15-4-1-2-7-18(15)16/h1-7,12-13H,8-11,14H2,(H,31,32) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444297
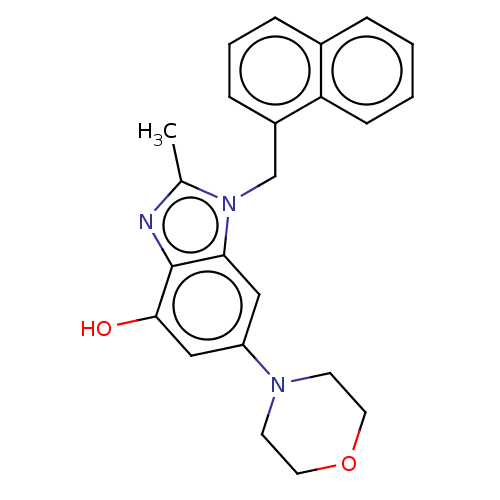
(US10660898, Example 5)Show InChI InChI=1S/C23H23N3O2/c1-16-24-23-21(13-19(14-22(23)27)25-9-11-28-12-10-25)26(16)15-18-7-4-6-17-5-2-3-8-20(17)18/h2-8,13-14,27H,9-12,15H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444321
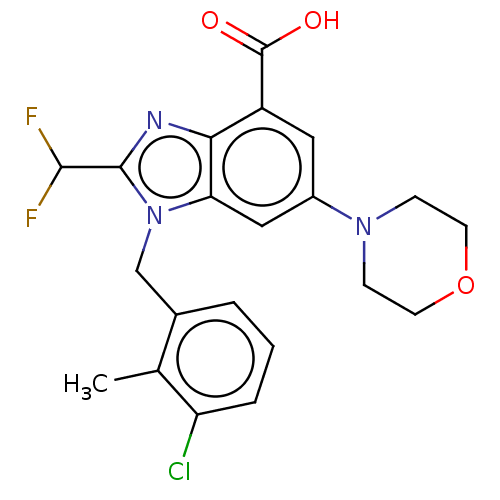
(US10660898, Example 72)Show SMILES Cc1c(Cl)cccc1Cn1c(nc2c(cc(cc12)N1CCOCC1)C(O)=O)C(F)F Show InChI InChI=1S/C21H20ClF2N3O3/c1-12-13(3-2-4-16(12)22)11-27-17-10-14(26-5-7-30-8-6-26)9-15(21(28)29)18(17)25-20(27)19(23)24/h2-4,9-10,19H,5-8,11H2,1H3,(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444312
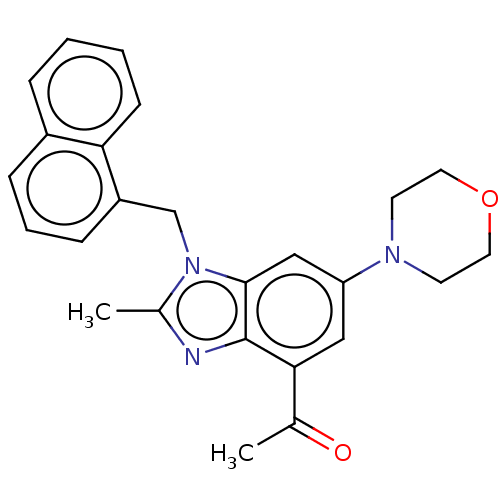
(US10660898, Example 41)Show SMILES CC(=O)c1cc(cc2n(Cc3cccc4ccccc34)c(C)nc12)N1CCOCC1 Show InChI InChI=1S/C25H25N3O2/c1-17(29)23-14-21(27-10-12-30-13-11-27)15-24-25(23)26-18(2)28(24)16-20-8-5-7-19-6-3-4-9-22(19)20/h3-9,14-15H,10-13,16H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444298
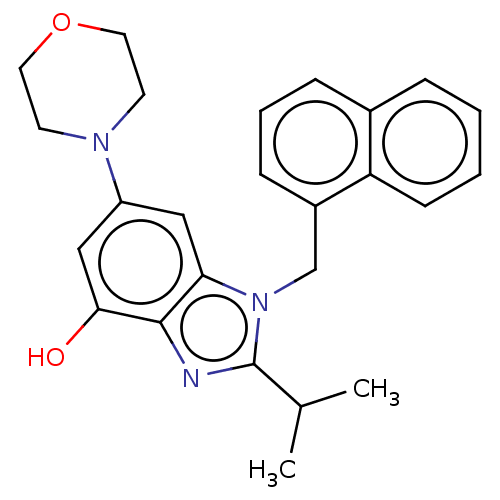
(US10660898, Example 11)Show SMILES CC(C)c1nc2c(O)cc(cc2n1Cc1cccc2ccccc12)N1CCOCC1 Show InChI InChI=1S/C25H27N3O2/c1-17(2)25-26-24-22(14-20(15-23(24)29)27-10-12-30-13-11-27)28(25)16-19-8-5-7-18-6-3-4-9-21(18)19/h3-9,14-15,17,29H,10-13,16H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444316
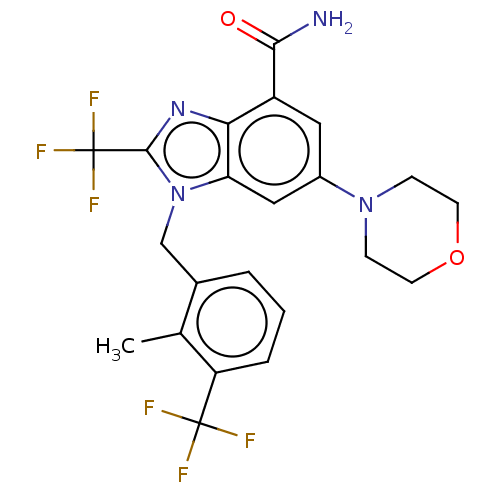
(US10660898, Example 54)Show SMILES Cc1c(Cn2c(nc3c(cc(cc23)N2CCOCC2)C(N)=O)C(F)(F)F)cccc1C(F)(F)F Show InChI InChI=1S/C22H20F6N4O2/c1-12-13(3-2-4-16(12)21(23,24)25)11-32-17-10-14(31-5-7-34-8-6-31)9-15(19(29)33)18(17)30-20(32)22(26,27)28/h2-4,9-10H,5-8,11H2,1H3,(H2,29,33) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444302

(US10660898, Example 17)Show SMILES CCc1nc2c(F)cc(cc2n1Cc1cccc2ccccc12)N1CCOCC1 Show InChI InChI=1S/C24H24FN3O/c1-2-23-26-24-21(25)14-19(27-10-12-29-13-11-27)15-22(24)28(23)16-18-8-5-7-17-6-3-4-9-20(17)18/h3-9,14-15H,2,10-13,16H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444300

(US10660898, Example 14)Show SMILES CCc1nc2c(O)cc(cc2n1Cc1cccc(Cl)c1Cl)N1CCOCC1 Show InChI InChI=1S/C20H21Cl2N3O2/c1-2-18-23-20-16(25(18)12-13-4-3-5-15(21)19(13)22)10-14(11-17(20)26)24-6-8-27-9-7-24/h3-5,10-11,26H,2,6-9,12H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50512861
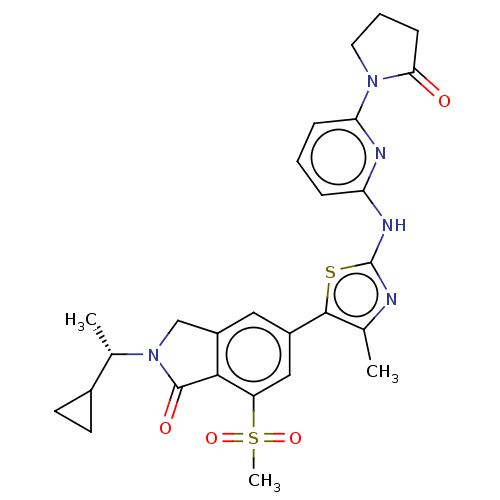
(CHEMBL4558527)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(Nc2cccc(n2)N2CCCC2=O)nc1C |r| Show InChI InChI=1S/C27H29N5O4S2/c1-15-25(37-27(28-15)30-21-6-4-7-22(29-21)31-11-5-8-23(31)33)18-12-19-14-32(16(2)17-9-10-17)26(34)24(19)20(13-18)38(3,35)36/h4,6-7,12-13,16-17H,5,8-11,14H2,1-3H3,(H,28,29,30)/t16-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIK3C2B (unknown origin) assessed as reduction in substrate phosphorylation by FRET Adapta assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444313
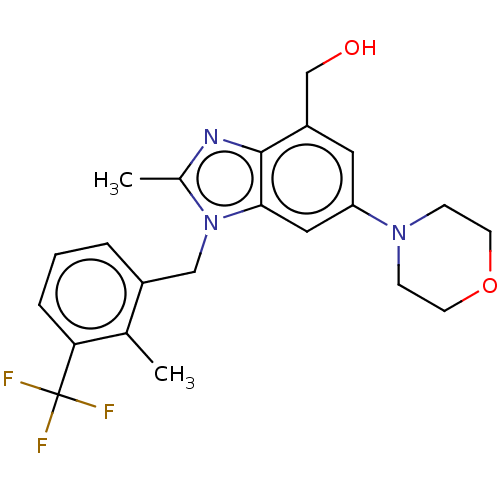
(US10660898, Example 43)Show SMILES Cc1nc2c(CO)cc(cc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C22H24F3N3O2/c1-14-16(4-3-5-19(14)22(23,24)25)12-28-15(2)26-21-17(13-29)10-18(11-20(21)28)27-6-8-30-9-7-27/h3-5,10-11,29H,6-9,12-13H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50059637
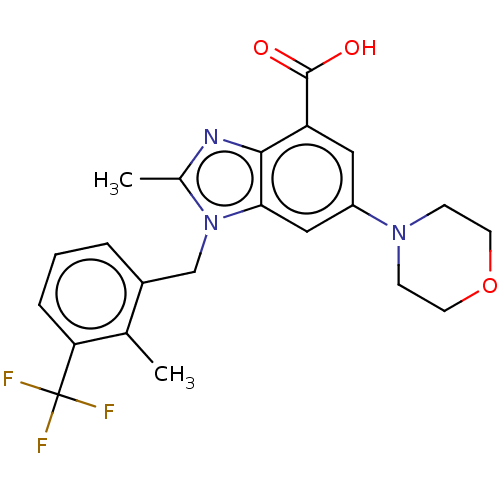
(GSK-2636771 | GSK2636771 | US10660898, Example 31)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1)C(O)=O Show InChI InChI=1S/C22H22F3N3O3/c1-13-15(4-3-5-18(13)22(23,24)25)12-28-14(2)26-20-17(21(29)30)10-16(11-19(20)28)27-6-8-31-9-7-27/h3-5,10-11H,6-9,12H2,1-2H3,(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444304
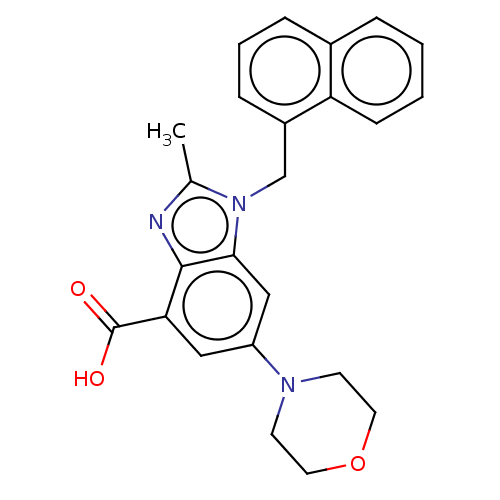
(US10660898, Example 20)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc2ccccc12)N1CCOCC1)C(O)=O Show InChI InChI=1S/C24H23N3O3/c1-16-25-23-21(24(28)29)13-19(26-9-11-30-12-10-26)14-22(23)27(16)15-18-7-4-6-17-5-2-3-8-20(17)18/h2-8,13-14H,9-12,15H2,1H3,(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444318

(US10660898, Example 59)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc(Cl)c1C)N1CCOCC1)C(O)=O Show InChI InChI=1S/C21H22ClN3O3/c1-13-15(4-3-5-18(13)22)12-25-14(2)23-20-17(21(26)27)10-16(11-19(20)25)24-6-8-28-9-7-24/h3-5,10-11H,6-9,12H2,1-2H3,(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444317
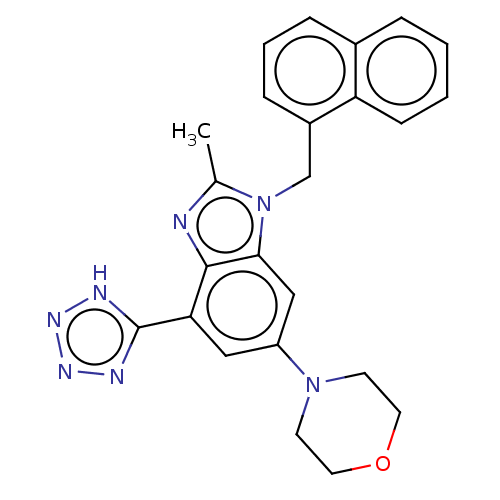
(US10660898, Example 58)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc2ccccc12)N1CCOCC1)-c1nnn[nH]1 Show InChI InChI=1S/C24H23N7O/c1-16-25-23-21(24-26-28-29-27-24)13-19(30-9-11-32-12-10-30)14-22(23)31(16)15-18-7-4-6-17-5-2-3-8-20(17)18/h2-8,13-14H,9-12,15H2,1H3,(H,26,27,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444299
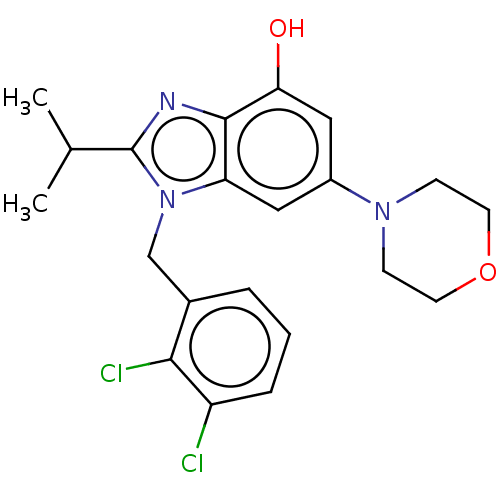
(US10660898, Example 13)Show SMILES CC(C)c1nc2c(O)cc(cc2n1Cc1cccc(Cl)c1Cl)N1CCOCC1 Show InChI InChI=1S/C21H23Cl2N3O2/c1-13(2)21-24-20-17(26(21)12-14-4-3-5-16(22)19(14)23)10-15(11-18(20)27)25-6-8-28-9-7-25/h3-5,10-11,13,27H,6-9,12H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444303
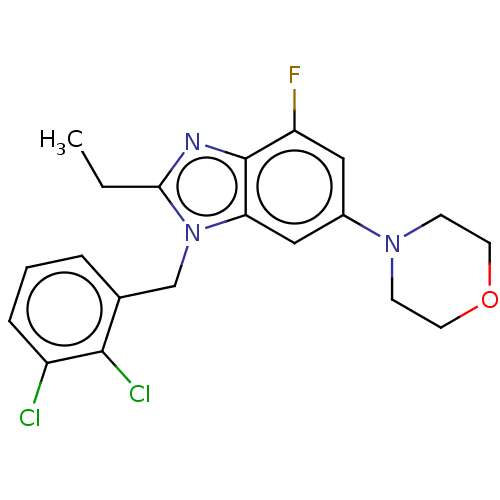
(US10660898, Example 18)Show SMILES CCc1nc2c(F)cc(cc2n1Cc1cccc(Cl)c1Cl)N1CCOCC1 Show InChI InChI=1S/C20H20Cl2FN3O/c1-2-18-24-20-16(23)10-14(25-6-8-27-9-7-25)11-17(20)26(18)12-13-4-3-5-15(21)19(13)22/h3-5,10-11H,2,6-9,12H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kc2beta |
Bioorg Med Chem Lett 17: 2438-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.032
BindingDB Entry DOI: 10.7270/Q28053F2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 5837-44 (2007)
Article DOI: 10.1016/j.bmc.2007.05.070
BindingDB Entry DOI: 10.7270/Q2X928M7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444308
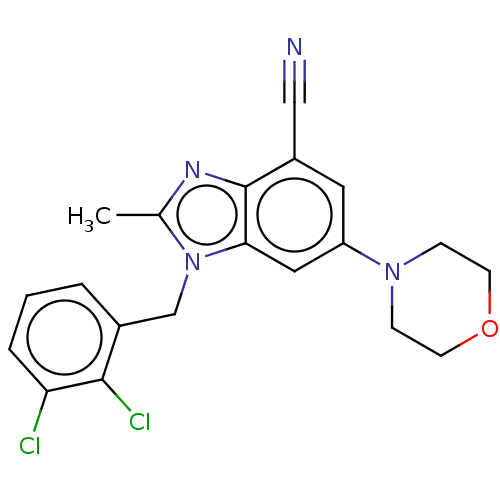
(US10660898, Example 32)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc(Cl)c1Cl)N1CCOCC1)C#N Show InChI InChI=1S/C20H18Cl2N4O/c1-13-24-20-15(11-23)9-16(25-5-7-27-8-6-25)10-18(20)26(13)12-14-3-2-4-17(21)19(14)22/h2-4,9-10H,5-8,12H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444305
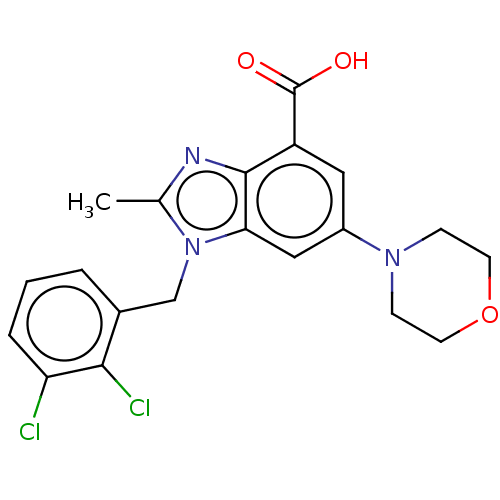
(US10660898, Example 22)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc(Cl)c1Cl)N1CCOCC1)C(O)=O Show InChI InChI=1S/C20H19Cl2N3O3/c1-12-23-19-15(20(26)27)9-14(24-5-7-28-8-6-24)10-17(19)25(12)11-13-3-2-4-16(21)18(13)22/h2-4,9-10H,5-8,11H2,1H3,(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50341209
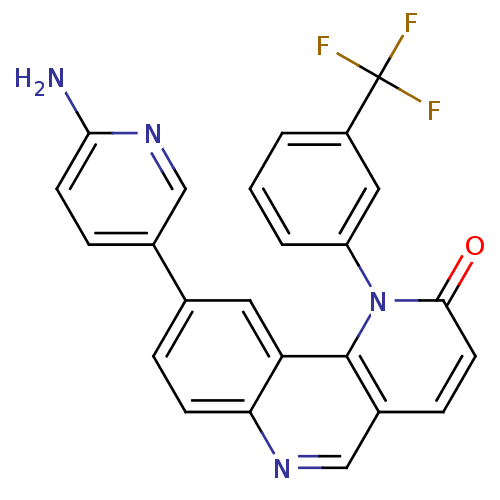
(9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phen...)Show SMILES Nc1ccc(cn1)-c1ccc2ncc3ccc(=O)n(-c4cccc(c4)C(F)(F)F)c3c2c1 Show InChI InChI=1S/C24H15F3N4O/c25-24(26,27)17-2-1-3-18(11-17)31-22(32)9-6-16-13-29-20-7-4-14(10-19(20)23(16)31)15-5-8-21(28)30-12-15/h1-13H,(H2,28,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-C2beta |
J Med Chem 54: 1473-80 (2011)
Article DOI: 10.1021/jm101520v
BindingDB Entry DOI: 10.7270/Q2S46S8H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444322

(US10660898, Example 73)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc2ccsc12)N1CCOCC1)C(O)=O Show InChI InChI=1S/C22H21N3O3S/c1-14-23-20-18(22(26)27)11-17(24-6-8-28-9-7-24)12-19(20)25(14)13-16-4-2-3-15-5-10-29-21(15)16/h2-5,10-12H,6-9,13H2,1H3,(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human PI3KC2beta by non-radiometric ADP-Glo assay |
ACS Med Chem Lett 6: 3-6 (2015)
Article DOI: 10.1021/ml500354e
BindingDB Entry DOI: 10.7270/Q2M61MWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50197065
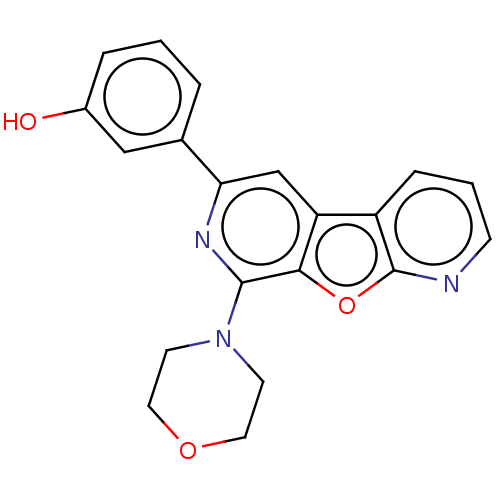
(CHEMBL3913354)Show InChI InChI=1S/C20H17N3O3/c24-14-4-1-3-13(11-14)17-12-16-15-5-2-6-21-20(15)26-18(16)19(22-17)23-7-9-25-10-8-23/h1-6,11-12,24H,7-10H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Curtin University
Curated by ChEMBL
| Assay Description
Inhibition of PI3KC2beta (unknown origin) expressed in HEK293 cells using phosphatidylinositol and gamma-32P-ATP incubated for 20 mins by TLC assay o... |
J Med Chem 60: 47-65 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00963
BindingDB Entry DOI: 10.7270/Q2PG1TPX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50394846
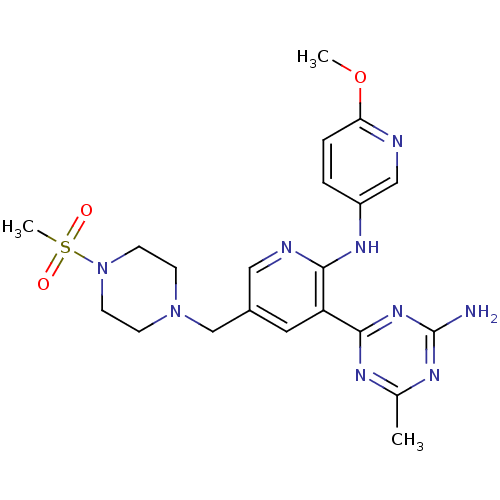
(CHEMBL2165017 | US8772480, 35)Show SMILES COc1ccc(Nc2ncc(CN3CCN(CC3)S(C)(=O)=O)cc2-c2nc(C)nc(N)n2)cn1 Show InChI InChI=1S/C21H27N9O3S/c1-14-25-20(28-21(22)26-14)17-10-15(13-29-6-8-30(9-7-29)34(3,31)32)11-24-19(17)27-16-4-5-18(33-2)23-12-16/h4-5,10-12H,6-9,13H2,1-3H3,(H,24,27)(H2,22,25,26,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PIK3C2beta |
J Med Chem 55: 5188-219 (2012)
Article DOI: 10.1021/jm300184s
BindingDB Entry DOI: 10.7270/Q23F4QSJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50274638
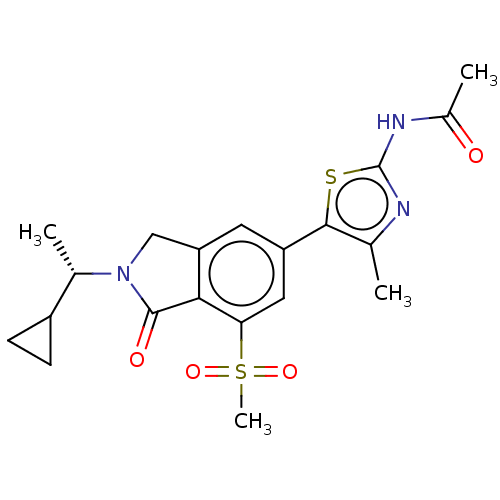
(CHEMBL4126156 | US10858355, Example 4)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O4S2/c1-10-18(28-20(21-10)22-12(3)24)14-7-15-9-23(11(2)13-5-6-13)19(25)17(15)16(8-14)29(4,26)27/h7-8,11,13H,5-6,9H2,1-4H3,(H,21,22,24)/t11-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3KC2beta (unknown origin) |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50042923
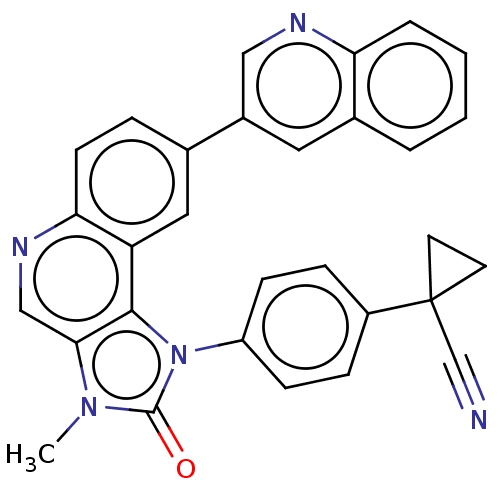
(CHEMBL3354566)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C2(CC2)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H21N5O/c1-34-27-17-33-26-11-6-19(21-14-20-4-2-3-5-25(20)32-16-21)15-24(26)28(27)35(29(34)36)23-9-7-22(8-10-23)30(18-31)12-13-30/h2-11,14-17H,12-13H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human PI3KC2beta by non-radiometric ADP-Glo assay |
ACS Med Chem Lett 6: 3-6 (2015)
Article DOI: 10.1021/ml500354e
BindingDB Entry DOI: 10.7270/Q2M61MWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Curtin University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3KC2beta using substrate PI incubated for 1 hr by Adapta kinase assay |
J Med Chem 60: 47-65 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00963
BindingDB Entry DOI: 10.7270/Q2PG1TPX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444309
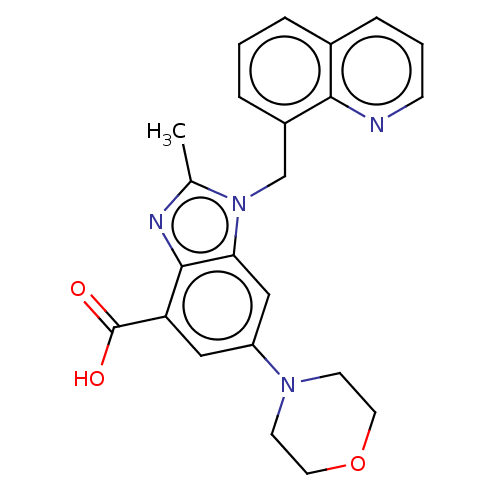
(US10660898, Example 35)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc2cccnc12)N1CCOCC1)C(O)=O Show InChI InChI=1S/C23H22N4O3/c1-15-25-22-19(23(28)29)12-18(26-8-10-30-11-9-26)13-20(22)27(15)14-17-5-2-4-16-6-3-7-24-21(16)17/h2-7,12-13H,8-11,14H2,1H3,(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM81368
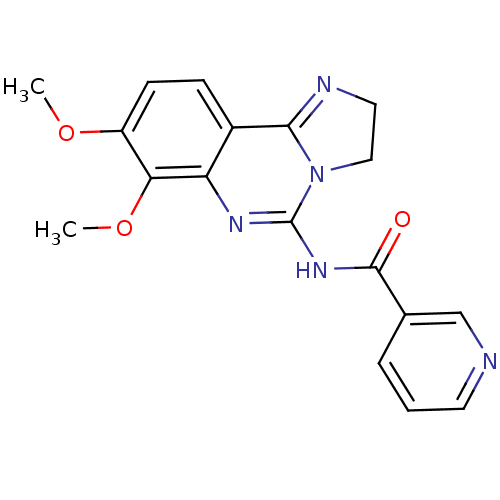
(PIK90)Show SMILES COc1ccc2C3=NCCN3C(NC(=O)c3cccnc3)=Nc2c1OC |c:22,t:6| Show InChI InChI=1S/C18H17N5O3/c1-25-13-6-5-12-14(15(13)26-2)21-18(23-9-8-20-16(12)23)22-17(24)11-4-3-7-19-10-11/h3-7,10H,8-9H2,1-2H3,(H,21,22,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human PI3KC2beta by non-radiometric ADP-Glo assay |
ACS Med Chem Lett 6: 3-6 (2015)
Article DOI: 10.1021/ml500354e
BindingDB Entry DOI: 10.7270/Q2M61MWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM81368

(PIK90)Show SMILES COc1ccc2C3=NCCN3C(NC(=O)c3cccnc3)=Nc2c1OC |c:22,t:6| Show InChI InChI=1S/C18H17N5O3/c1-25-13-6-5-12-14(15(13)26-2)21-18(23-9-8-20-16(12)23)22-17(24)11-4-3-7-19-10-11/h3-7,10H,8-9H2,1-2H3,(H,21,22,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Curtin University
Curated by ChEMBL
| Assay Description
Inhibition of PI3KC2beta (unknown origin) expressed in HEK293 cells using phosphatidylinositol and gamma-32P-ATP incubated for 20 mins by TLC assay o... |
J Med Chem 60: 47-65 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00963
BindingDB Entry DOI: 10.7270/Q2PG1TPX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM348119
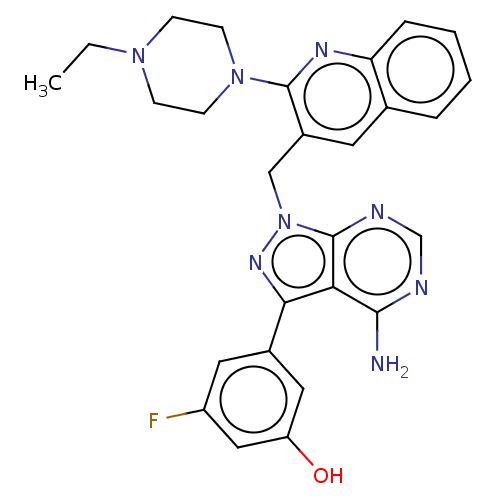
(US9790228, Compound 38)Show SMILES CCN1CCN(CC1)c1nc2ccccc2cc1Cn1nc(-c2cc(O)cc(F)c2)c2c(N)ncnc12 Show InChI InChI=1S/C27H27FN8O/c1-2-34-7-9-35(10-8-34)26-19(11-17-5-3-4-6-22(17)32-26)15-36-27-23(25(29)30-16-31-27)24(33-36)18-12-20(28)14-21(37)13-18/h3-6,11-14,16,37H,2,7-10,15H2,1H3,(H2,29,30,31) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Intellikine LLC
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9790228 (2017)
BindingDB Entry DOI: 10.7270/Q26112FV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM348131

(US9790228, Compound 197 | US9790228, Compound 50)Show SMILES CN1CCN(CC1)c1nc2ccccc2cc1Cn1nc(-c2cc(O)cc(F)c2)c2c(N)ncnc12 Show InChI InChI=1S/C26H25FN8O/c1-33-6-8-34(9-7-33)25-18(10-16-4-2-3-5-21(16)31-25)14-35-26-22(24(28)29-15-30-26)23(32-35)17-11-19(27)13-20(36)12-17/h2-5,10-13,15,36H,6-9,14H2,1H3,(H2,28,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Intellikine LLC
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9790228 (2017)
BindingDB Entry DOI: 10.7270/Q26112FV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM444310
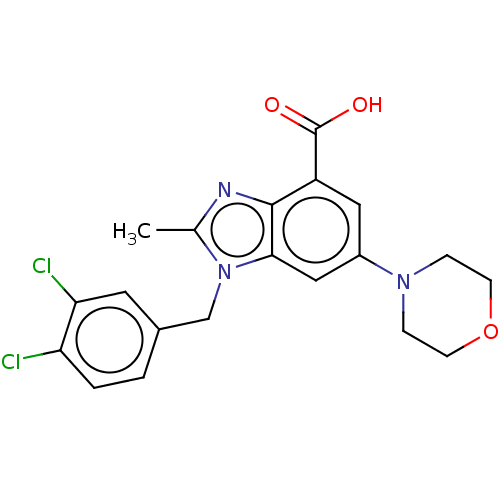
(US10660898, Example 38)Show SMILES Cc1nc2c(cc(cc2n1Cc1ccc(Cl)c(Cl)c1)N1CCOCC1)C(O)=O Show InChI InChI=1S/C20H19Cl2N3O3/c1-12-23-19-15(20(26)27)9-14(24-4-6-28-7-5-24)10-18(19)25(12)11-13-2-3-16(21)17(22)8-13/h2-3,8-10H,4-7,11H2,1H3,(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GLAXOSMITHKLINE LLC
US Patent
| Assay Description
PI3Kinase Reaction Buffer was prepared by diluting the stock 1:4 with de-ionized water. Freshly prepared DTT was added at a final concentration of 5 ... |
US Patent US10660898 (2020)
BindingDB Entry DOI: 10.7270/Q20V8GTT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM348255
(US9790228, Compound 174)Show SMILES Nc1ncnc2n(Cc3cc4ccccc4nc3CN3CCCC3)nc(-c3cc(O)cc(F)c3)c12 Show InChI InChI=1S/C26H24FN7O/c27-19-10-17(11-20(35)12-19)24-23-25(28)29-15-30-26(23)34(32-24)13-18-9-16-5-1-2-6-21(16)31-22(18)14-33-7-3-4-8-33/h1-2,5-6,9-12,15,35H,3-4,7-8,13-14H2,(H2,28,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Intellikine LLC
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9790228 (2017)
BindingDB Entry DOI: 10.7270/Q26112FV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50323731
(CHEMBL1213117 | N-(6-(4-amino-1-((2-(4-methylpiper...)Show SMILES CN1CCN(CC1)c1nc2ccccc2cc1Cn1nc(-c2ccc3nc(NC(C)=O)sc3c2)c2c(N)ncnc12 Show InChI InChI=1S/C29H28N10OS/c1-17(40)33-29-35-22-8-7-19(14-23(22)41-29)25-24-26(30)31-16-32-28(24)39(36-25)15-20-13-18-5-3-4-6-21(18)34-27(20)38-11-9-37(2)10-12-38/h3-8,13-14,16H,9-12,15H2,1-2H3,(H2,30,31,32)(H,33,35,40) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Intellikine LLC
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9790228 (2017)
BindingDB Entry DOI: 10.7270/Q26112FV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM348245
(US9790228, Compound 164)Show SMILES CC(C)N1CCN(CC1)c1nc2ccccc2cc1Cn1nc(-c2ccc3nc(N)sc3c2)c2c(N)ncnc12 Show InChI InChI=1S/C29H30N10S/c1-17(2)37-9-11-38(12-10-37)27-20(13-18-5-3-4-6-21(18)34-27)15-39-28-24(26(30)32-16-33-28)25(36-39)19-7-8-22-23(14-19)40-29(31)35-22/h3-8,13-14,16-17H,9-12,15H2,1-2H3,(H2,31,35)(H2,30,32,33) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Intellikine LLC
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9790228 (2017)
BindingDB Entry DOI: 10.7270/Q26112FV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM25036
(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 5837-44 (2007)
Article DOI: 10.1016/j.bmc.2007.05.070
BindingDB Entry DOI: 10.7270/Q2X928M7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM348244
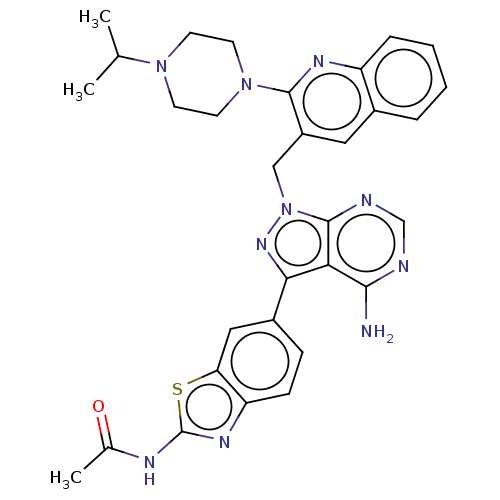
(US9790228, Compound 163)Show SMILES CC(C)N1CCN(CC1)c1nc2ccccc2cc1Cn1nc(-c2ccc3nc(NC(C)=O)sc3c2)c2c(N)ncnc12 Show InChI InChI=1S/C31H32N10OS/c1-18(2)39-10-12-40(13-11-39)29-22(14-20-6-4-5-7-23(20)36-29)16-41-30-26(28(32)33-17-34-30)27(38-41)21-8-9-24-25(15-21)43-31(37-24)35-19(3)42/h4-9,14-15,17-18H,10-13,16H2,1-3H3,(H2,32,33,34)(H,35,37,42) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Intellikine LLC
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9790228 (2017)
BindingDB Entry DOI: 10.7270/Q26112FV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM25056
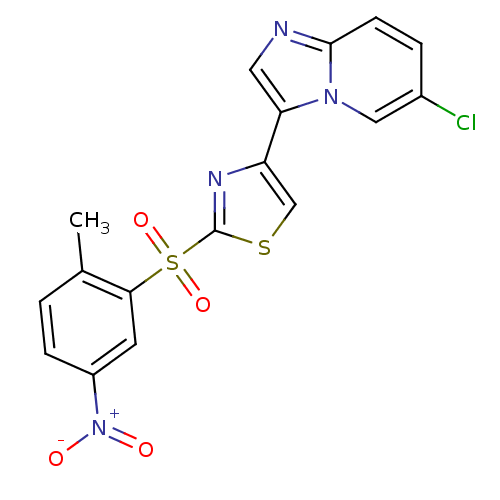
(4-{6-chloroimidazo[1,2-a]pyridin-3-yl}-2-[(2-methy...)Show SMILES Cc1ccc(cc1S(=O)(=O)c1nc(cs1)-c1cnc2ccc(Cl)cn12)[N+]([O-])=O Show InChI InChI=1S/C17H11ClN4O4S2/c1-10-2-4-12(22(23)24)6-15(10)28(25,26)17-20-13(9-27-17)14-7-19-16-5-3-11(18)8-21(14)16/h2-9H,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM348220

(US9790228, Compound 139)Show SMILES Nc1ncc2cc(ccc2n1)-c1nn(Cc2cc3ccccc3nc2N2CCOCC2)c2ncnc(N)c12 Show InChI InChI=1S/C27H24N10O/c28-24-22-23(17-5-6-21-18(12-17)13-30-27(29)34-21)35-37(26(22)32-15-31-24)14-19-11-16-3-1-2-4-20(16)33-25(19)36-7-9-38-10-8-36/h1-6,11-13,15H,7-10,14H2,(H2,28,31,32)(H2,29,30,34) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Intellikine LLC
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9790228 (2017)
BindingDB Entry DOI: 10.7270/Q26112FV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM348211
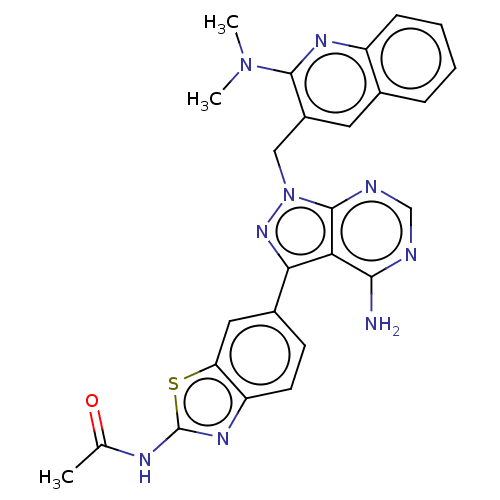
(US9790228, Compound 130)Show SMILES CN(C)c1nc2ccccc2cc1Cn1nc(-c2ccc3nc(NC(C)=O)sc3c2)c2c(N)ncnc12 Show InChI InChI=1S/C26H23N9OS/c1-14(36)30-26-32-19-9-8-16(11-20(19)37-26)22-21-23(27)28-13-29-25(21)35(33-22)12-17-10-15-6-4-5-7-18(15)31-24(17)34(2)3/h4-11,13H,12H2,1-3H3,(H2,27,28,29)(H,30,32,36) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Intellikine LLC
US Patent
| Assay Description
Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ... |
US Patent US9790228 (2017)
BindingDB Entry DOI: 10.7270/Q26112FV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data