

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bromodomain-containing protein 4 [333-460] (Homo sapiens (Human)) | BDBM205431 ((6S+2S)-PEG1 (7)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute | Assay Description Refer to DiscoverX | Nat Chem Biol 12: 1089-1096 (2016) Article DOI: 10.1038/nchembio.2209 BindingDB Entry DOI: 10.7270/Q2VQ31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform C of Bromodomain-containing protein 4 (Short) (Homo sapiens (Human)) | BDBM205431 ((6S+2S)-PEG1 (7)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute | Assay Description Refer to DiscoverX | Nat Chem Biol 12: 1089-1096 (2016) Article DOI: 10.1038/nchembio.2209 BindingDB Entry DOI: 10.7270/Q2VQ31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [44-168,333-460] (Homo sapiens (Human)) | BDBM205431 ((6S+2S)-PEG1 (7)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute | Assay Description Refer to DiscoverX | Nat Chem Biol 12: 1089-1096 (2016) Article DOI: 10.1038/nchembio.2209 BindingDB Entry DOI: 10.7270/Q2VQ31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [333-460] (Homo sapiens (Human)) | BDBM205453 (MTI (35)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute | Assay Description Refer to DiscoverX | Nat Chem Biol 12: 1089-1096 (2016) Article DOI: 10.1038/nchembio.2209 BindingDB Entry DOI: 10.7270/Q2VQ31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform C of Bromodomain-containing protein 4 (Short) (Homo sapiens (Human)) | BDBM205453 (MTI (35)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute | Assay Description Refer to DiscoverX | Nat Chem Biol 12: 1089-1096 (2016) Article DOI: 10.1038/nchembio.2209 BindingDB Entry DOI: 10.7270/Q2VQ31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [44-168,333-460] (Homo sapiens (Human)) | BDBM205453 (MTI (35)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute | Assay Description Refer to DiscoverX | Nat Chem Biol 12: 1089-1096 (2016) Article DOI: 10.1038/nchembio.2209 BindingDB Entry DOI: 10.7270/Q2VQ31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [44-168] (Homo sapiens (Human)) | BDBM205453 (MTI (35)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute | Assay Description Refer to DiscoverX | Nat Chem Biol 12: 1089-1096 (2016) Article DOI: 10.1038/nchembio.2209 BindingDB Entry DOI: 10.7270/Q2VQ31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [44-168] (Homo sapiens (Human)) | BDBM205431 ((6S+2S)-PEG1 (7)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute | Assay Description Refer to DiscoverX | Nat Chem Biol 12: 1089-1096 (2016) Article DOI: 10.1038/nchembio.2209 BindingDB Entry DOI: 10.7270/Q2VQ31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220432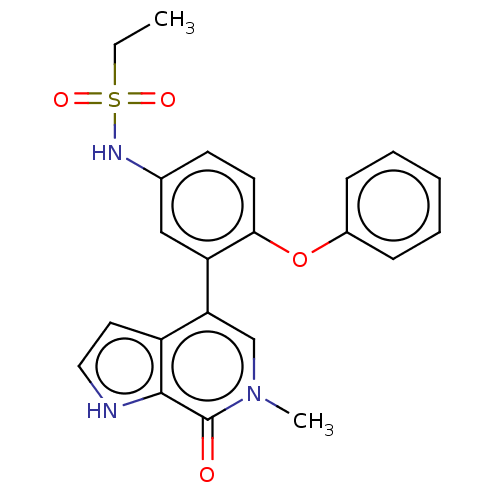 (US9296741, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.25 | -54.8 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220432 (US9296741, 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.390 | -53.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM439528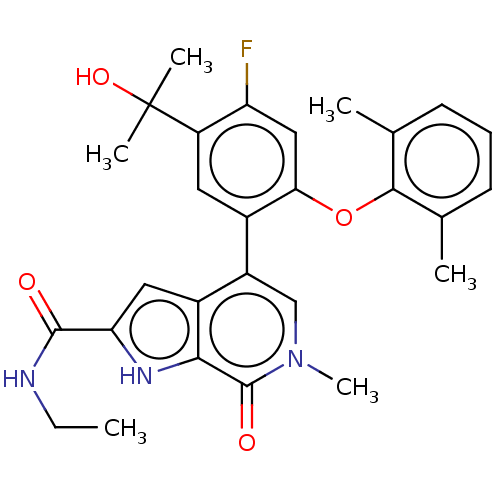 (US10633379, Example 64) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description Compound dilution series were prepared in DMSO via an approximately 3-fold serial dilution. Compound dilutions were added directly into white, low-vo... | US Patent US10633379 (2020) BindingDB Entry DOI: 10.7270/Q2PR801W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220626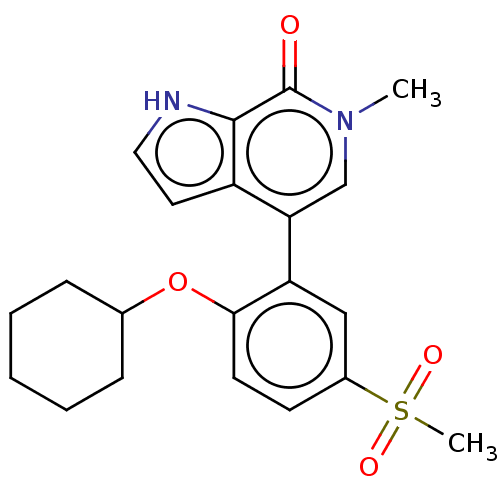 (US9296741, 215) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.460 | -53.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220518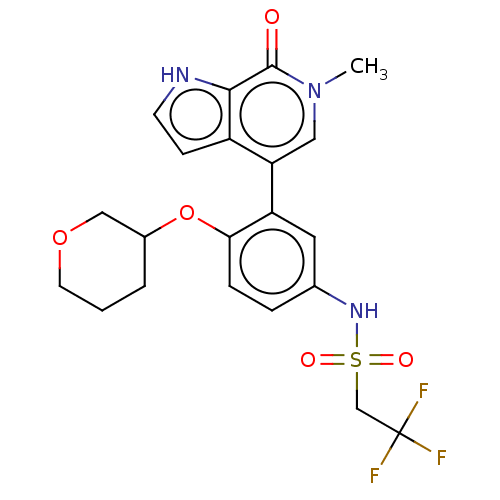 (US9296741, 107) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50459819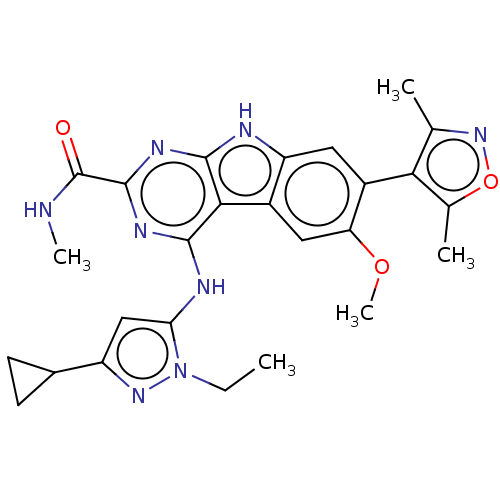 (CHEMBL4228445) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) (unknown origin) expressed in ... | J Med Chem 61: 462-481 (2018) Article DOI: 10.1021/acs.jmedchem.6b01816 BindingDB Entry DOI: 10.7270/Q2CV4MD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511850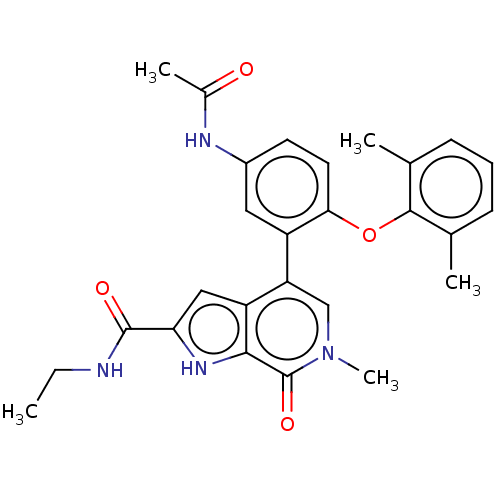 (CHEMBL4435166) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM439590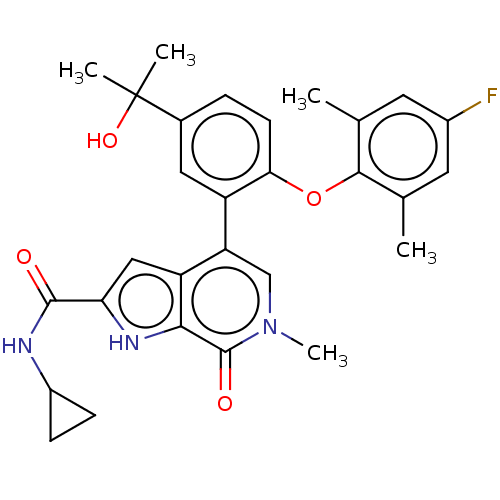 (US10633379, Example 121) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366704 (CHEMBL4166630) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453810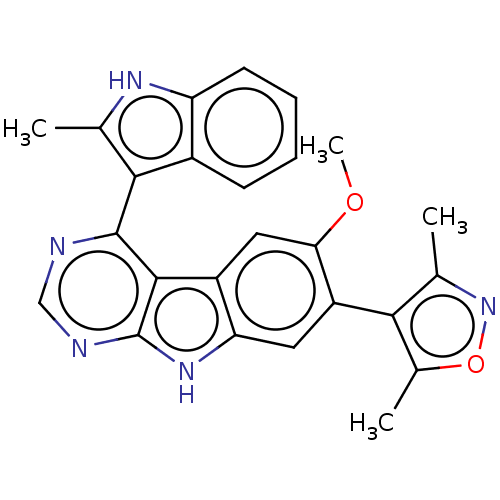 (CHEMBL4208405) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM179480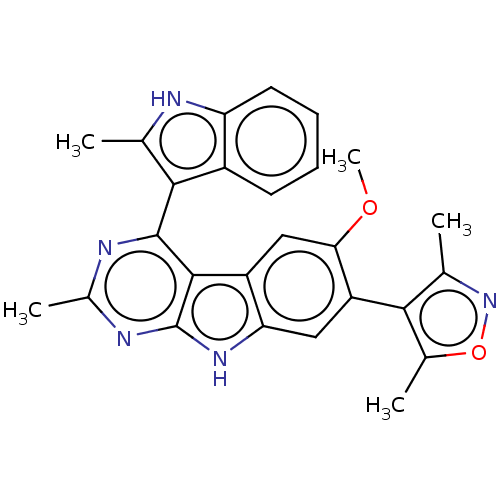 (US9675697, Cpd. No. 68) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 2 (333 to 460 residues) expressed in Rosetta2 D... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511875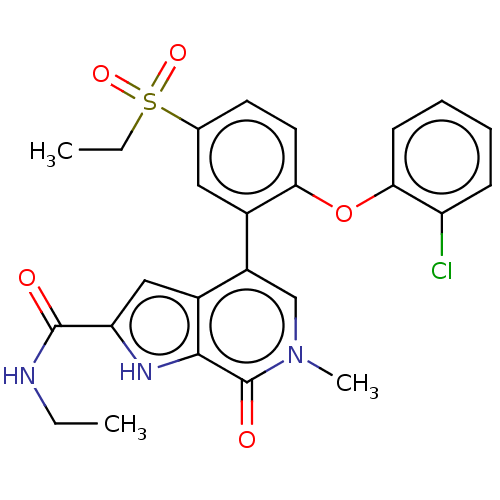 (CHEMBL4548794) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453807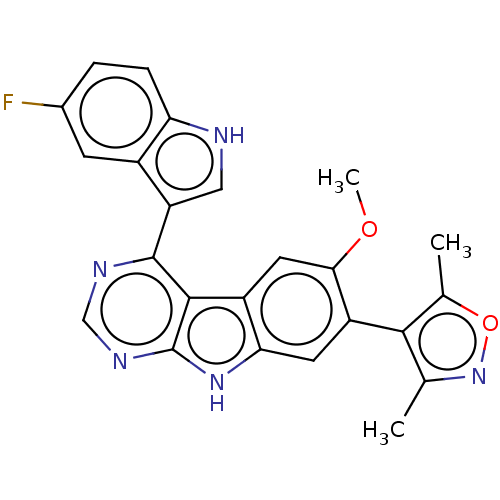 (CHEMBL4211497) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511849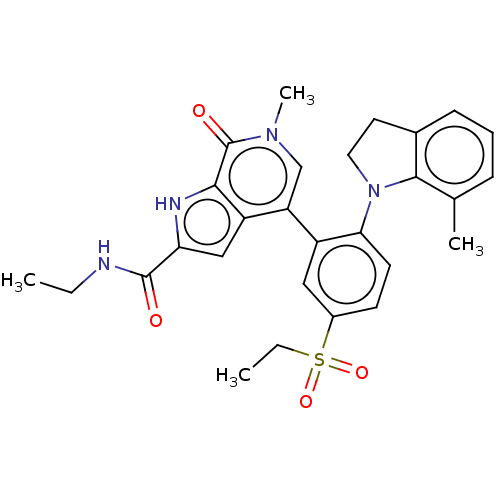 (CHEMBL4465299) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453769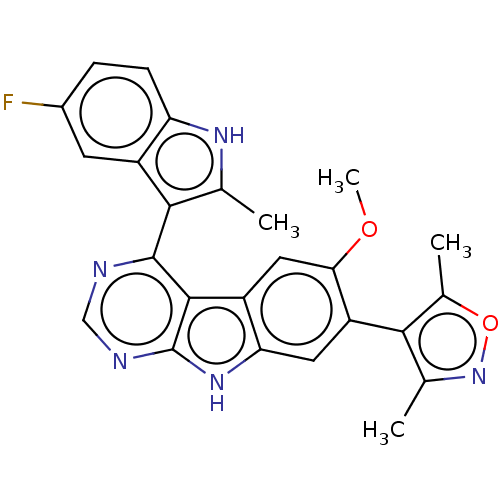 (CHEMBL4214978) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 DE... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220484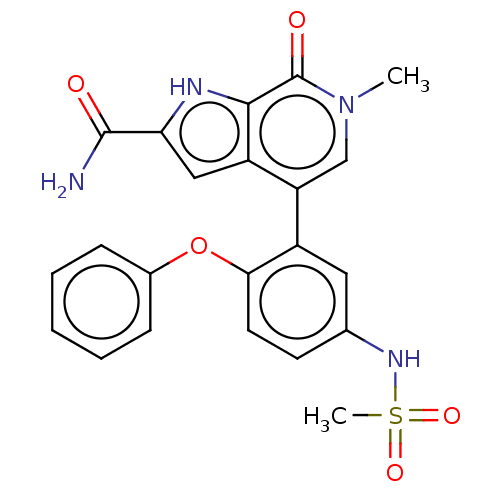 (US9296741, 73) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220452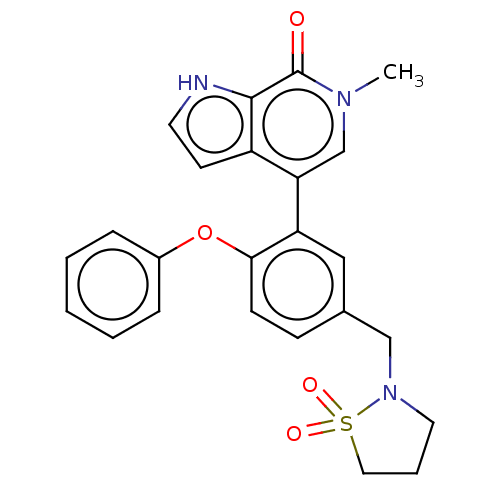 (US9296741, 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.510 | -53.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220516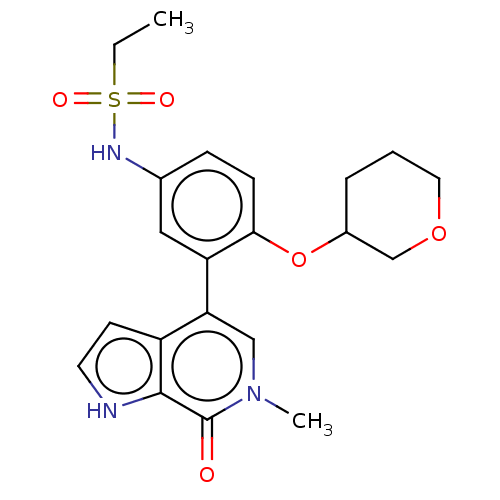 (US9296741, 105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.520 | -53.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220679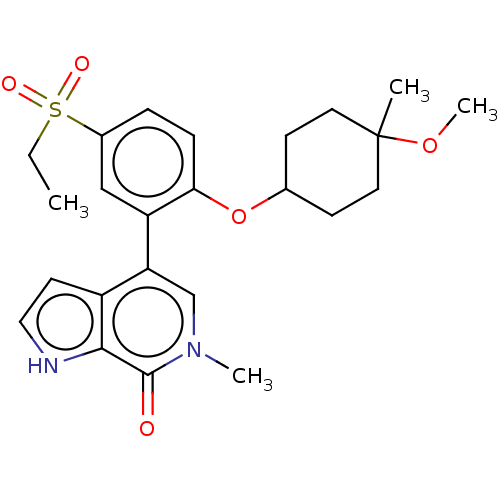 (US9296741, 268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | -52.8 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50260067 (CHEMBL4078267 | US9957263, Example 78) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Biology Program, Department of Pharmacology and Toxicology,?Department of Internal Medicine,žSealy Center for Molecular Medicine,?Institute for Translational Sciences, University of Texas Me Curated by ChEMBL | Assay Description Inhibition of His-tagged BRD4 bromodomain 2 (E352 to E168 residues) (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 60: 4533-4558 (2017) Article DOI: 10.1021/acs.jmedchem.6b01761 BindingDB Entry DOI: 10.7270/Q27P91VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50260083 (CHEMBL4104956) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Biology Program, Department of Pharmacology and Toxicology,?Department of Internal Medicine,žSealy Center for Molecular Medicine,?Institute for Translational Sciences, University of Texas Me Curated by ChEMBL | Assay Description Inhibition of His-tagged BRD4 bromodomain 2 (E352 to E168 residues) (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 60: 4533-4558 (2017) Article DOI: 10.1021/acs.jmedchem.6b01761 BindingDB Entry DOI: 10.7270/Q27P91VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM439590 (US10633379, Example 121) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description Compound dilution series were prepared in DMSO via an approximately 3-fold serial dilution. Compound dilutions were added directly into white, low-vo... | US Patent US10633379 (2020) BindingDB Entry DOI: 10.7270/Q2PR801W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50260067 (CHEMBL4078267 | US9957263, Example 78) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Compound dilution series were prepared in DMSO via a 3-fold serial dilution from 2.5 mM to 42 nM. Compounds were then diluted 6:100 in assay buffer (... | Bioorg Med Chem Lett 19: 360-4 (2009) BindingDB Entry DOI: 10.7270/Q2Q81GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511864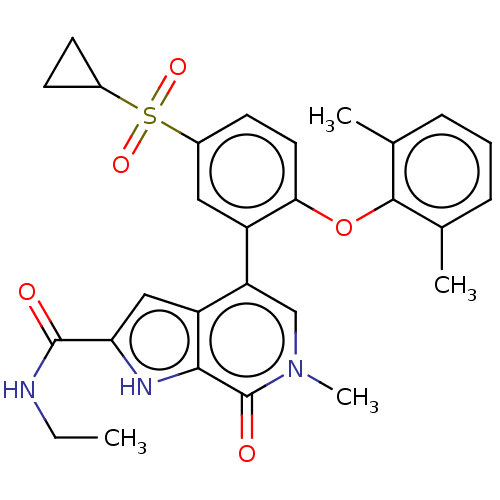 (CHEMBL4564879) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM439461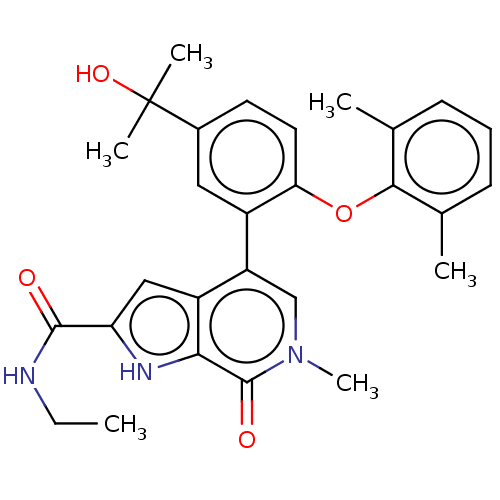 (US10633379, Example 3 | US10633379, Example 68) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM390899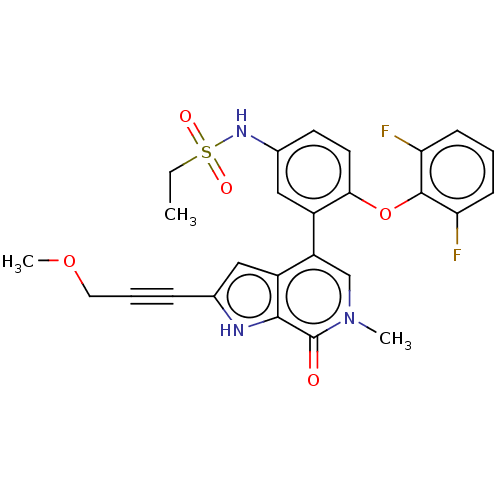 (N-{4-(2,4-difluorophenoxy)-3-[2-(3-methoxyprop-1-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.605 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Compound dilution series were prepared in DMSO via a 3-fold serial dilution from 2.5 mM to 42 nM. Compounds were then diluted 6:100 in assay buffer (... | Bioorg Med Chem Lett 19: 360-4 (2009) BindingDB Entry DOI: 10.7270/Q2Q81GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50457489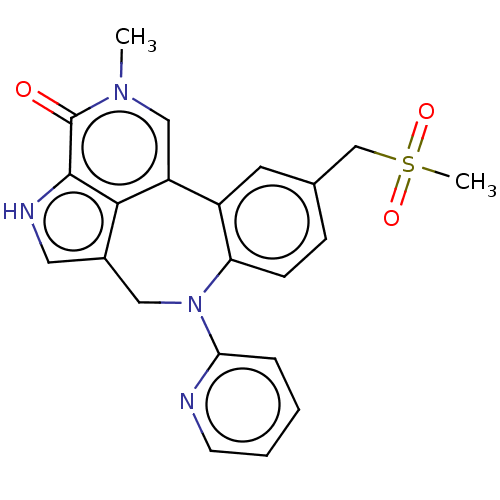 (CHEMBL4208129) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to His-tagged BRD4 bromodomain 1 to 2 (K57 to K550 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220507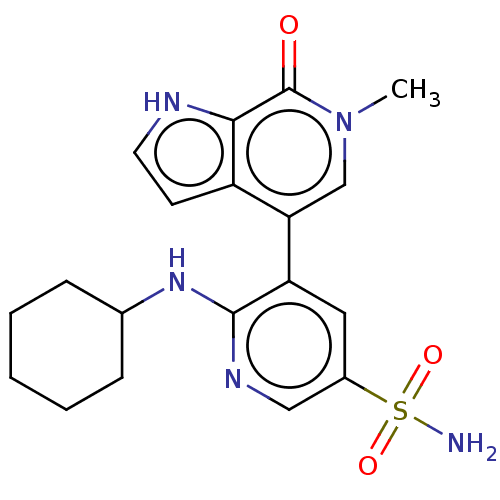 (US9296741, 96) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.630 | -52.5 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220495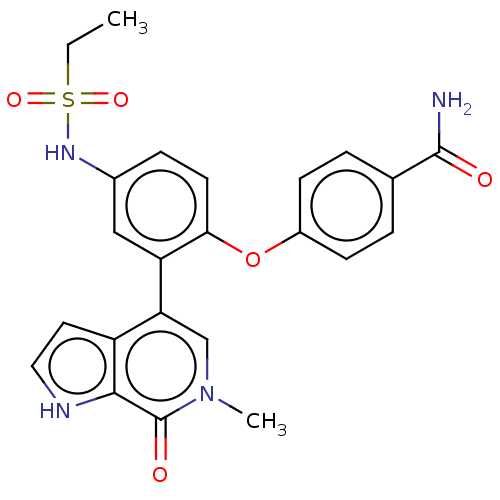 (US9296741, 84) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.650 | -52.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50156345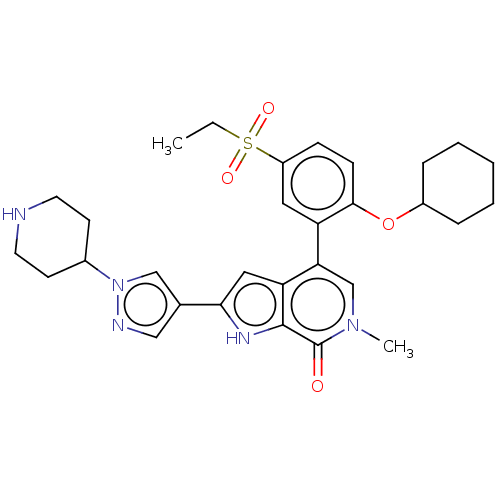 (CHEMBL3787680 | US9957263, Example 149) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.669 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Compound dilution series were prepared in DMSO via a 3-fold serial dilution from 2.5 mM to 42 nM. Compounds were then diluted 6:100 in assay buffer (... | Bioorg Med Chem Lett 19: 360-4 (2009) BindingDB Entry DOI: 10.7270/Q2Q81GDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220431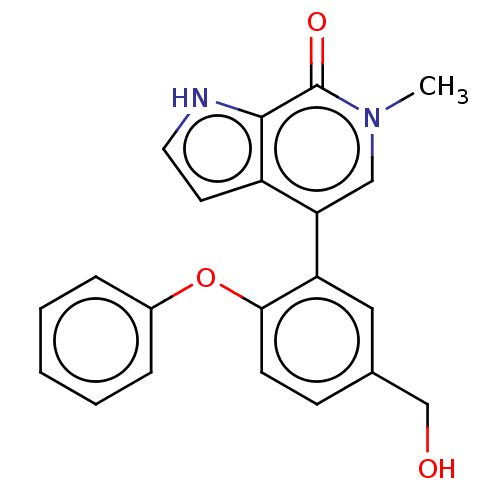 (US10633379, Compound Z | US9296741, 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.680 | -52.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220614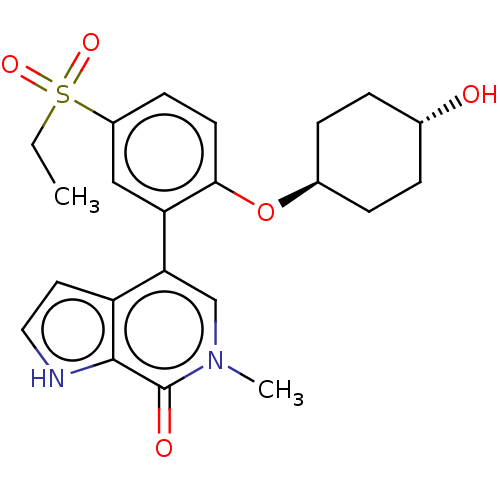 (US9296741, 203) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.690 | -52.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50453810 (CHEMBL4208405) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human N-terminal His6-tagged BRD4 bromodomain 2 (333 to 460 residues) expressed in Rosetta2 D... | J Med Chem 60: 3887-3901 (2017) Article DOI: 10.1021/acs.jmedchem.7b00193 BindingDB Entry DOI: 10.7270/Q28W3GWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511855 (CHEMBL4454597) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511870 (CHEMBL4461291) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50366716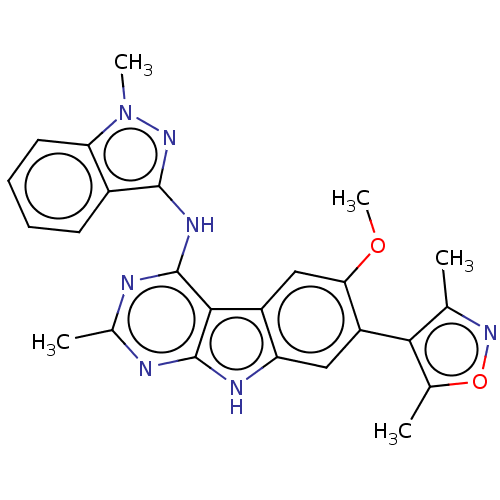 (CHEMBL4174669) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FAM-labeled ZBA248 binding to recombinant human BRD4 bromodomain 1 (44 to 168 residues) expressed in Rosetta2 Escherichia coli DE3 cell... | J Med Chem 61: 6110-6120 (2018) Article DOI: 10.1021/acs.jmedchem.8b00483 BindingDB Entry DOI: 10.7270/Q26M39B1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220520 (US9296741, 109) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.720 | -52.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50457496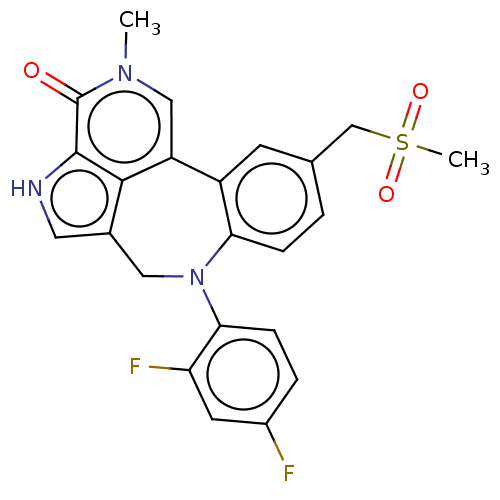 (CHEMBL4217457) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to BRD4 bromodomain 2 (E352 to M457 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by bromo... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220477 (US9296741, 66) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.740 | -52.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220434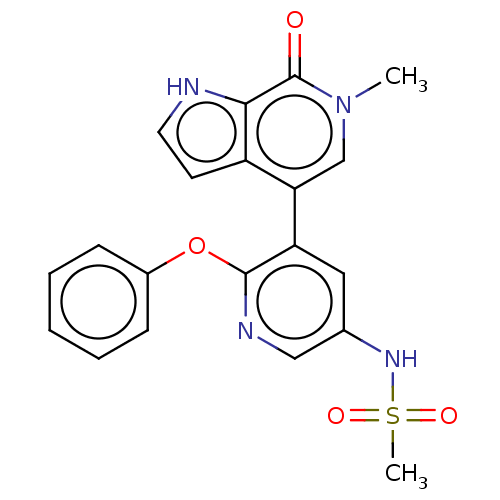 (US9296741, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.75 | -52.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220614 (US9296741, 203) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.75 | -52.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220471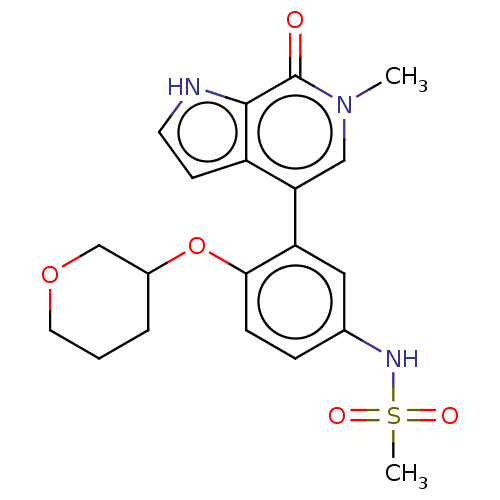 (US9296741, 60) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.75 | -52.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 21444 total ) | Next | Last >> |