

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50331094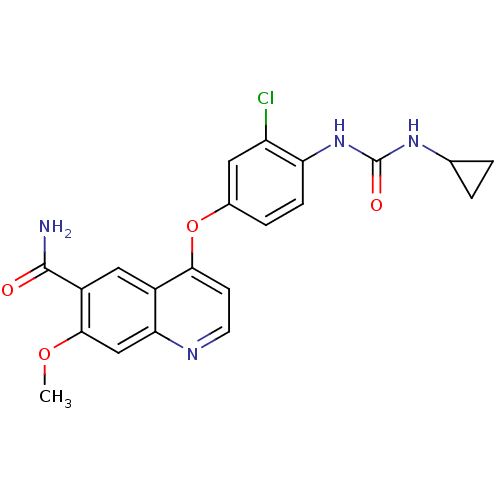 (4-(3-chloro-4-(3-cyclopropylureido)phenoxy)-7-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation (DGMIF) Curated by ChEMBL | Assay Description Inhibition of RET (unknown origin) | J Med Chem 58: 3672-81 (2015) Article DOI: 10.1021/jm501464c BindingDB Entry DOI: 10.7270/Q2RR2106 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50020310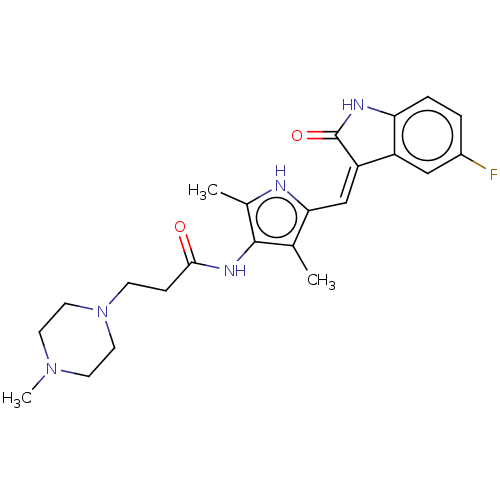 (CHEMBL3288854) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qilu Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Binding affinity to RET (unknown origin) | Eur J Med Chem 82: 139-51 (2014) Article DOI: 10.1016/j.ejmech.2014.05.051 BindingDB Entry DOI: 10.7270/Q2F1918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50198782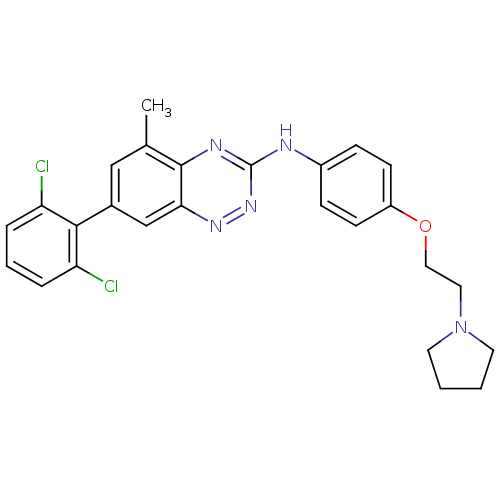 (([7-(2,6-dichloro-phenyl)-5-methyl-benzo[1,2,4]tri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc. Curated by ChEMBL | Assay Description Inhibition of Ret | Bioorg Med Chem Lett 17: 602-8 (2007) Article DOI: 10.1016/j.bmcl.2006.11.006 BindingDB Entry DOI: 10.7270/Q29024MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50341519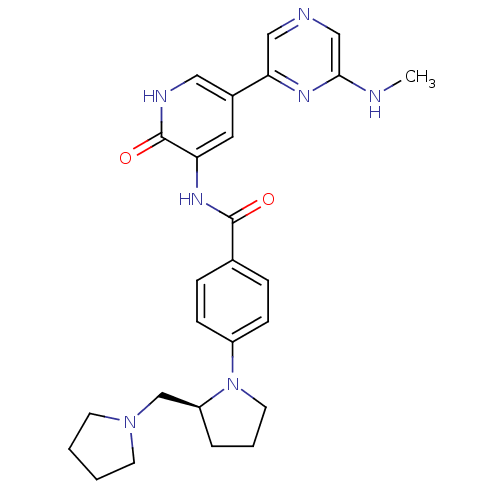 ((S)-3-(4-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of RET | J Med Chem 54: 2341-50 (2011) Article DOI: 10.1021/jm101499u BindingDB Entry DOI: 10.7270/Q2KH0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50463483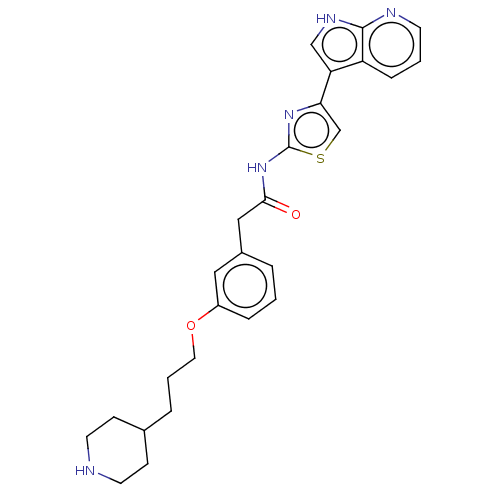 (CHEMBL4245242) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of RET (unknown origin) | Bioorg Med Chem Lett 28: 2622-2626 (2018) Article DOI: 10.1016/j.bmcl.2018.06.040 BindingDB Entry DOI: 10.7270/Q2ZC85HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50463479 (CHEMBL4249925) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of RET (unknown origin) | Bioorg Med Chem Lett 28: 2622-2626 (2018) Article DOI: 10.1016/j.bmcl.2018.06.040 BindingDB Entry DOI: 10.7270/Q2ZC85HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret [G810S] (Homo sapiens (Human)) | BDBM576988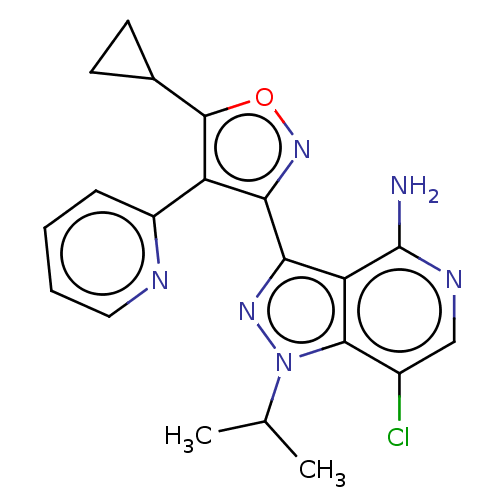 (US11472802, Example 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55HG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret [V804M] (Homo sapiens (Human)) | BDBM576988 (US11472802, Example 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55HG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM576988 (US11472802, Example 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370388 (US10233189, Example 81 | US10787457, Example 81 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2B85BDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50240779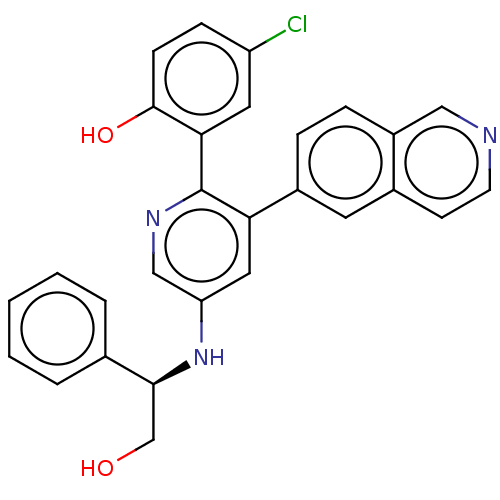 (CHEMBL4067871) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant Ret (unknown origin) using poly (Glu,Tyr) 4:1 as substrate after 60 mins by ELISA | J Med Chem 60: 6018-6035 (2017) Article DOI: 10.1021/acs.jmedchem.7b00076 BindingDB Entry DOI: 10.7270/Q2K939NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370388 (US10233189, Example 81 | US10787457, Example 81 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154M86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370388 (US10233189, Example 81 | US10787457, Example 81 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | US Patent US10787457 (2020) BindingDB Entry DOI: 10.7270/Q2BR8W77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM499158 (US11014930, Example 81) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | US Patent US11014930 (2021) BindingDB Entry DOI: 10.7270/Q26D5X4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370382 (US10233189, Example 79 | US10787457, Example 79 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2B85BDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370382 (US10233189, Example 79 | US10787457, Example 79 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154M86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM499156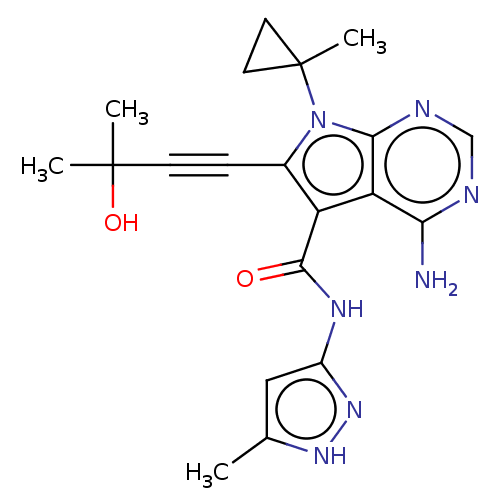 (US11014930, Example 79) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | US Patent US11014930 (2021) BindingDB Entry DOI: 10.7270/Q26D5X4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370382 (US10233189, Example 79 | US10787457, Example 79 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | US Patent US10787457 (2020) BindingDB Entry DOI: 10.7270/Q2BR8W77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370385 (US10233189, Example 80 | US10787457, Example 80 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2B85BDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370385 (US10233189, Example 80 | US10787457, Example 80 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154M86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM499157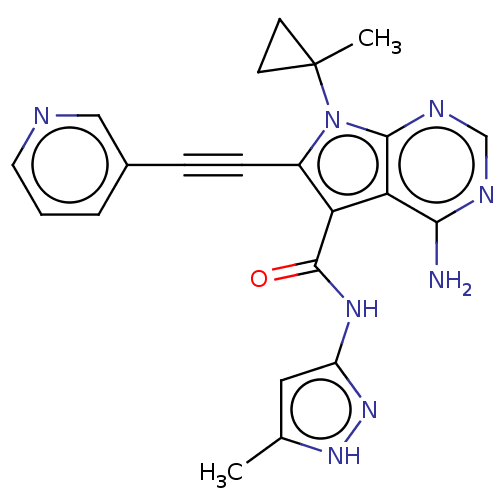 (US11014930, Example 80) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | US Patent US11014930 (2021) BindingDB Entry DOI: 10.7270/Q26D5X4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370385 (US10233189, Example 80 | US10787457, Example 80 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | US Patent US10787457 (2020) BindingDB Entry DOI: 10.7270/Q2BR8W77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM311639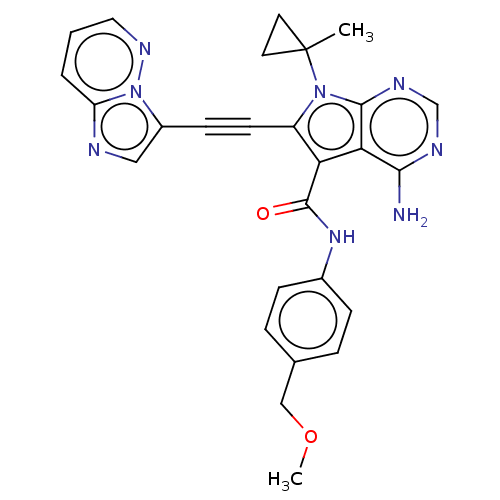 (Ex. Cpd. 91 | US10807986, Example 91 | US11046696,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | US Patent US10155768 (2018) BindingDB Entry DOI: 10.7270/Q2X350J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM577053 (US11472802, Example 58) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM311705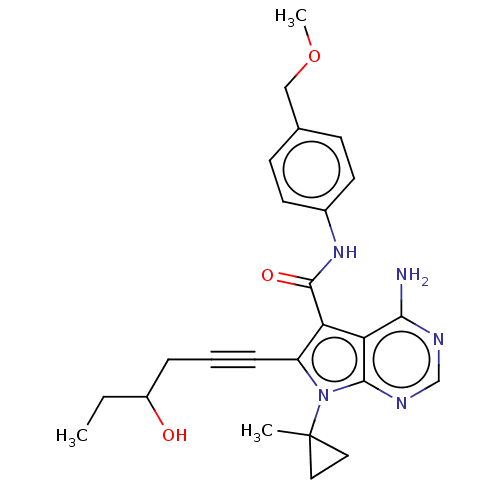 (Ex. Cpd. 157 | US10807986, Example 157 | US1104669...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | US Patent US10155768 (2018) BindingDB Entry DOI: 10.7270/Q2X350J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM311622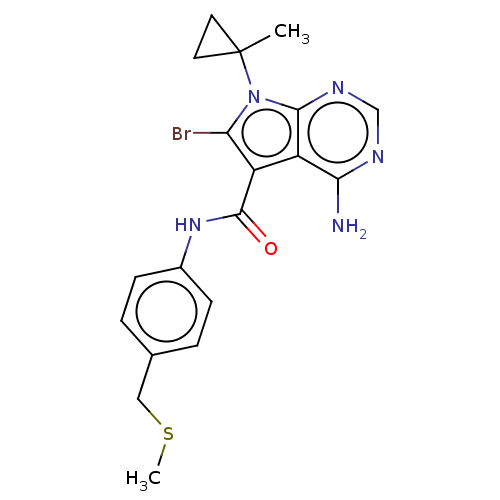 (Ex. Cpd. 59 | US10807986, Example 59 | US11046696,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | US Patent US10155768 (2018) BindingDB Entry DOI: 10.7270/Q2X350J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370397 (US10233189, Example 84 | US10787457, Example 84 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2B85BDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370400 (US10233189, Example 85 | US10787457, Example 85 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2B85BDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM311679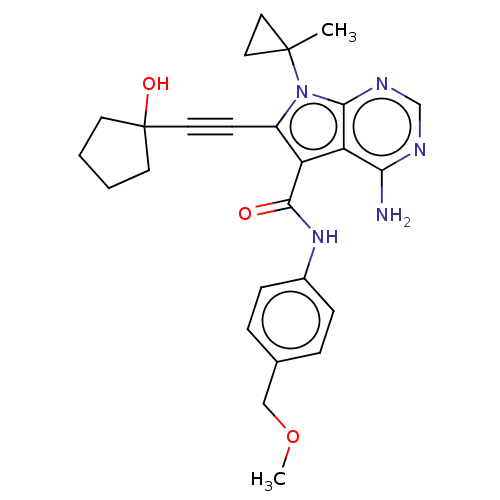 (Ex. Cpd. 131 | US10807986, Example 131 | US1104669...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | US Patent US10807986 (2020) BindingDB Entry DOI: 10.7270/Q29G5QWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM311622 (Ex. Cpd. 59 | US10807986, Example 59 | US11046696,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | US Patent US10807986 (2020) BindingDB Entry DOI: 10.7270/Q29G5QWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50458625 (CHEMBL4218013) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human RET using cisbio TK biotin-peptide as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by HT... | ACS Med Chem Lett 9: 623-628 (2018) Article DOI: 10.1021/acsmedchemlett.8b00035 BindingDB Entry DOI: 10.7270/Q2TB19GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM311705 (Ex. Cpd. 157 | US10807986, Example 157 | US1104669...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | US Patent US10807986 (2020) BindingDB Entry DOI: 10.7270/Q29G5QWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM311639 (Ex. Cpd. 91 | US10807986, Example 91 | US11046696,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | US Patent US10807986 (2020) BindingDB Entry DOI: 10.7270/Q29G5QWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM499161 (US11014930, Example 84) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | US Patent US11014930 (2021) BindingDB Entry DOI: 10.7270/Q26D5X4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370400 (US10233189, Example 85 | US10787457, Example 85 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | US Patent US11014930 (2021) BindingDB Entry DOI: 10.7270/Q26D5X4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370397 (US10233189, Example 84 | US10787457, Example 84 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | US Patent US10787457 (2020) BindingDB Entry DOI: 10.7270/Q2BR8W77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370400 (US10233189, Example 85 | US10787457, Example 85 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | US Patent US10787457 (2020) BindingDB Entry DOI: 10.7270/Q2BR8W77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM311679 (Ex. Cpd. 131 | US10807986, Example 131 | US1104669...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | Citation and Details BindingDB Entry DOI: 10.7270/Q2280BR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM311705 (Ex. Cpd. 157 | US10807986, Example 157 | US1104669...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | Citation and Details BindingDB Entry DOI: 10.7270/Q2280BR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM311622 (Ex. Cpd. 59 | US10807986, Example 59 | US11046696,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | Citation and Details BindingDB Entry DOI: 10.7270/Q2280BR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM311639 (Ex. Cpd. 91 | US10807986, Example 91 | US11046696,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | Citation and Details BindingDB Entry DOI: 10.7270/Q2280BR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret [G810R] (Homo sapiens (Human)) | BDBM576988 (US11472802, Example 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22N55HG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370397 (US10233189, Example 84 | US10787457, Example 84 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154M86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370400 (US10233189, Example 85 | US10787457, Example 85 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154M86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM311679 (Ex. Cpd. 131 | US10807986, Example 131 | US1104669...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | US Patent US10155768 (2018) BindingDB Entry DOI: 10.7270/Q2X350J3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50595124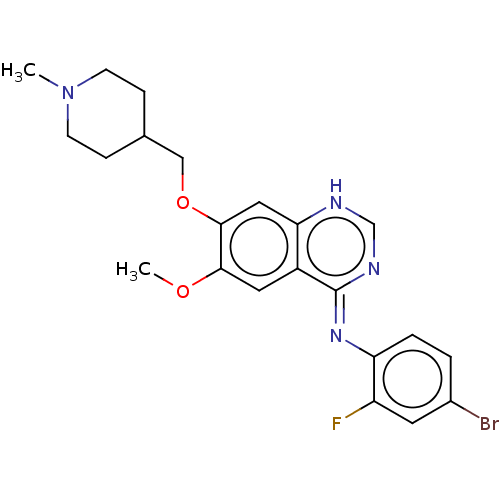 (CHEBI:49960 | Caprelsa | GNF-PF-2188 | NSC-744325 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02167 BindingDB Entry DOI: 10.7270/Q22R3WQ4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370372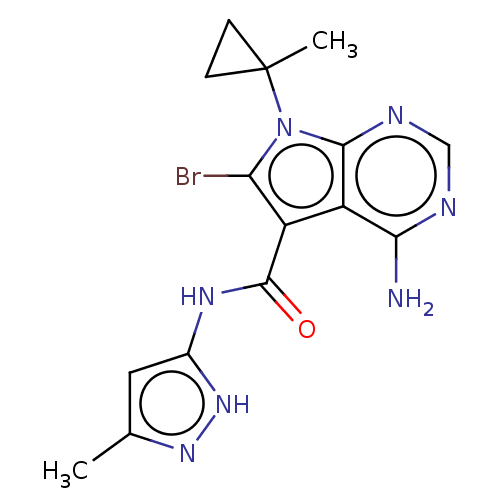 (US10233189, Example 76 | US10787457, Example 76 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q2B85BDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370372 (US10233189, Example 76 | US10787457, Example 76 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Regarding the conditions for measurement of in vitro inhibitory activity of compounds against RET kinase activity, the website of AnaSpec states that... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154M86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM499153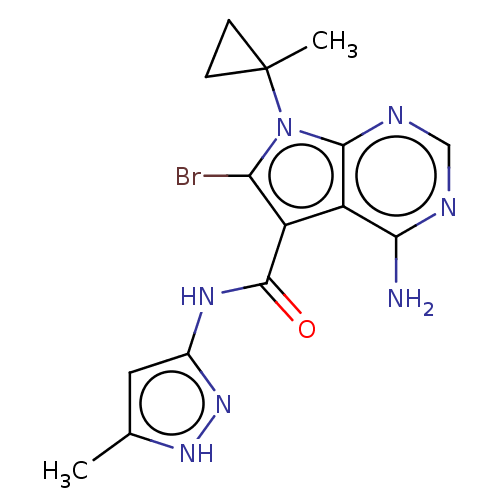 (US11014930, Example 76) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | US Patent US11014930 (2021) BindingDB Entry DOI: 10.7270/Q26D5X4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM370372 (US10233189, Example 76 | US10787457, Example 76 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description To measure the inhibitory activity, first, the compounds of the present invention were individually diluted with dimethyl sulfoxide (DMSO) stepwise. ... | US Patent US10787457 (2020) BindingDB Entry DOI: 10.7270/Q2BR8W77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 26585 total ) | Next | Last >> |