

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50524696 (CHEMBL4571835) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry | Eur J Med Chem 177: 247-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.051 BindingDB Entry DOI: 10.7270/Q24M980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50448370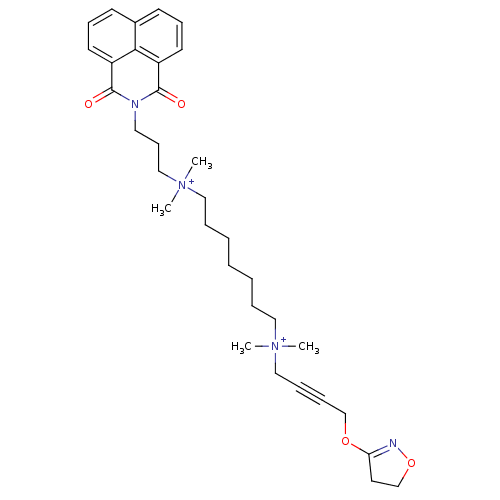 (CHEMBL3120178) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in rat brain homogenate after 15 mins by Ellman assay | Eur J Med Chem 75: 222-32 (2014) Article DOI: 10.1016/j.ejmech.2014.01.032 BindingDB Entry DOI: 10.7270/Q2K075RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50524701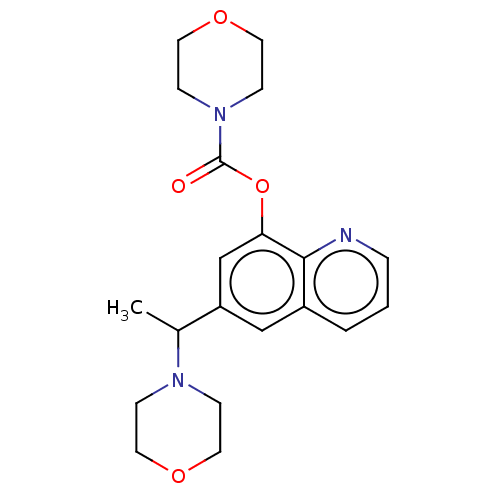 (CHEMBL4594074) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry | Eur J Med Chem 177: 247-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.051 BindingDB Entry DOI: 10.7270/Q24M980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50524708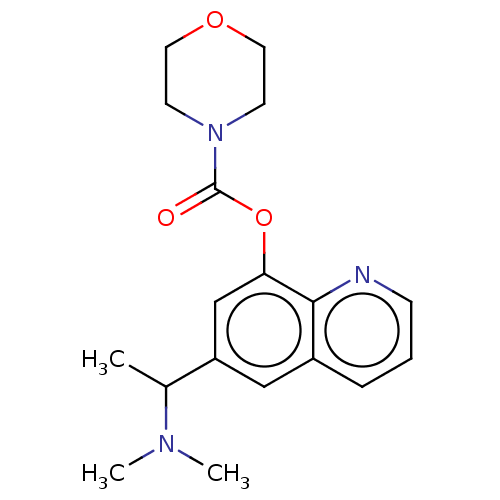 (CHEMBL4459614) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry | Eur J Med Chem 177: 247-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.051 BindingDB Entry DOI: 10.7270/Q24M980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry | Eur J Med Chem 177: 247-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.051 BindingDB Entry DOI: 10.7270/Q24M980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50448375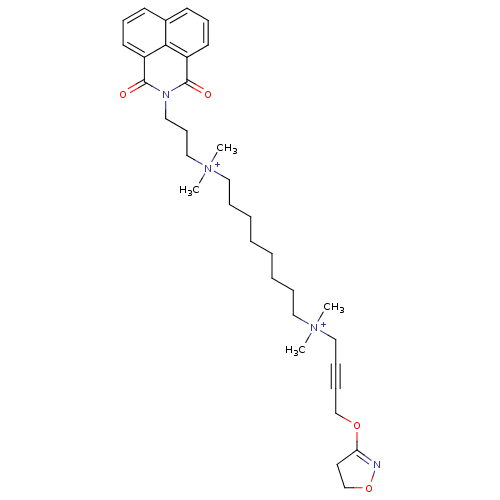 (CHEMBL3121479) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in rat brain homogenate after 15 mins by Ellman assay | Eur J Med Chem 75: 222-32 (2014) Article DOI: 10.1016/j.ejmech.2014.01.032 BindingDB Entry DOI: 10.7270/Q2K075RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50448374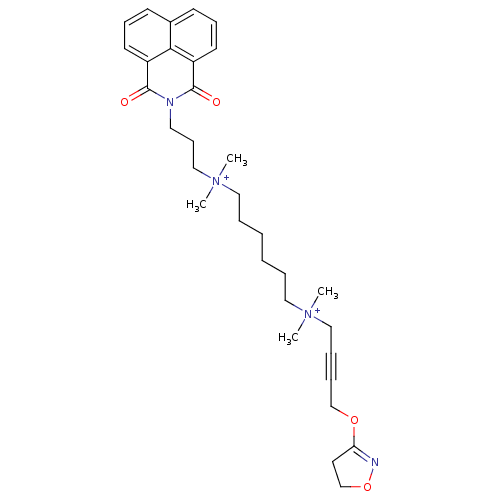 (CHEMBL3121478) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in rat brain homogenate after 15 mins by Ellman assay | Eur J Med Chem 75: 222-32 (2014) Article DOI: 10.1016/j.ejmech.2014.01.032 BindingDB Entry DOI: 10.7270/Q2K075RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50524697 (CHEMBL4470950) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry | Eur J Med Chem 177: 247-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.051 BindingDB Entry DOI: 10.7270/Q24M980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50524707 (CHEMBL4468053) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry | Eur J Med Chem 177: 247-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.051 BindingDB Entry DOI: 10.7270/Q24M980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50524705 (CHEMBL4452617) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry | Eur J Med Chem 177: 247-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.051 BindingDB Entry DOI: 10.7270/Q24M980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50448369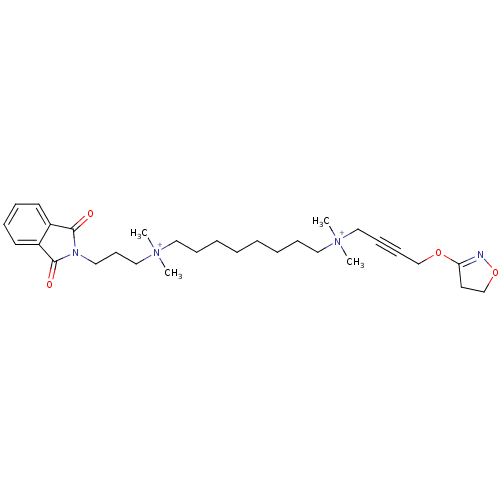 (CHEMBL3121476) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in rat brain homogenate after 15 mins by Ellman assay | Eur J Med Chem 75: 222-32 (2014) Article DOI: 10.1016/j.ejmech.2014.01.032 BindingDB Entry DOI: 10.7270/Q2K075RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50524698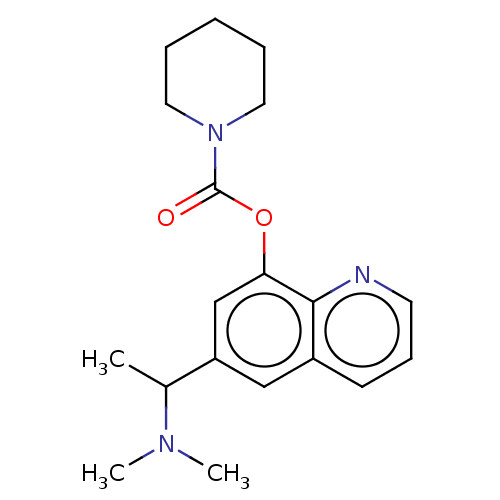 (CHEMBL4452986) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry | Eur J Med Chem 177: 247-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.051 BindingDB Entry DOI: 10.7270/Q24M980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50448371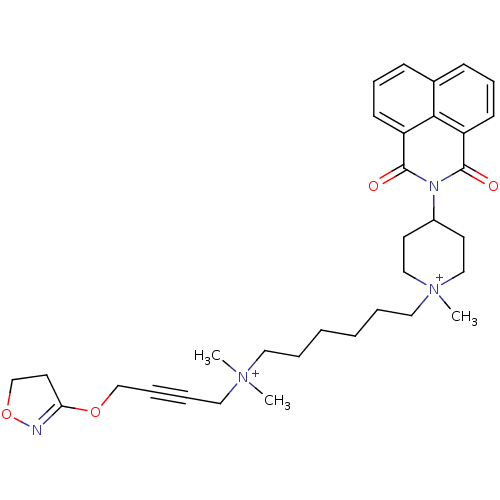 (CHEMBL3121480) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in rat brain homogenate after 15 mins by Ellman assay | Eur J Med Chem 75: 222-32 (2014) Article DOI: 10.1016/j.ejmech.2014.01.032 BindingDB Entry DOI: 10.7270/Q2K075RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50524706 (CHEMBL4566337) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry | Eur J Med Chem 177: 247-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.051 BindingDB Entry DOI: 10.7270/Q24M980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50524703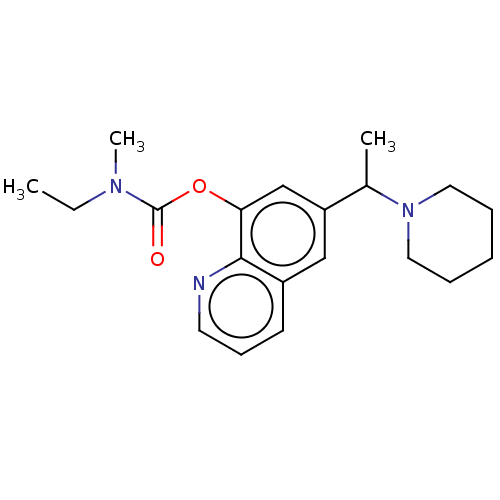 (CHEMBL4443818) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry | Eur J Med Chem 177: 247-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.051 BindingDB Entry DOI: 10.7270/Q24M980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50448372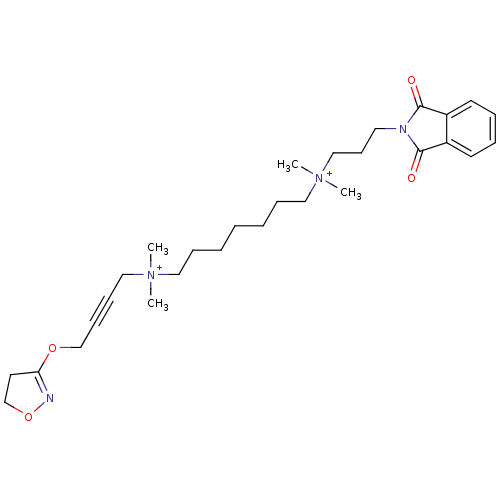 (CHEMBL3121475) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in rat brain homogenate after 15 mins by Ellman assay | Eur J Med Chem 75: 222-32 (2014) Article DOI: 10.1016/j.ejmech.2014.01.032 BindingDB Entry DOI: 10.7270/Q2K075RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50524709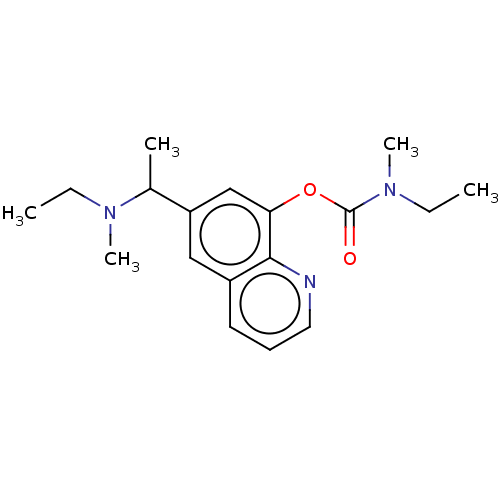 (CHEMBL4471688) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry | Eur J Med Chem 177: 247-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.051 BindingDB Entry DOI: 10.7270/Q24M980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50524702 (CHEMBL4517494) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry | Eur J Med Chem 177: 247-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.051 BindingDB Entry DOI: 10.7270/Q24M980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50448376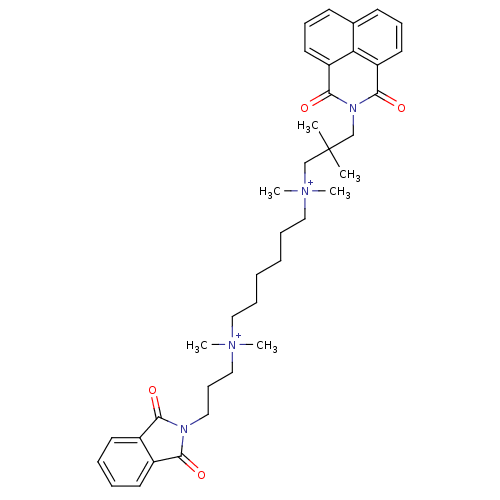 (Naphmethonium) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in rat brain homogenate after 15 mins by Ellman assay | Eur J Med Chem 75: 222-32 (2014) Article DOI: 10.1016/j.ejmech.2014.01.032 BindingDB Entry DOI: 10.7270/Q2K075RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50524700 (CHEMBL4535921) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry | Eur J Med Chem 177: 247-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.051 BindingDB Entry DOI: 10.7270/Q24M980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50524704 (CHEMBL4446271) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry | Eur J Med Chem 177: 247-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.051 BindingDB Entry DOI: 10.7270/Q24M980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Rattus norvegicus) | BDBM50524699 (CHEMBL4557064) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry | Eur J Med Chem 177: 247-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.051 BindingDB Entry DOI: 10.7270/Q24M980S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/epsilon (Rattus norvegicus-RAT) | BDBM50206243 (CHEMBL3918431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at rat alpha1beta1delta1epsilon nAChR expressed in HEK293 cells assessed as increase in Ca2+ flux by Fluo-4 AM dye based FLIPR assay | ACS Med Chem Lett 8: 366-371 (2017) Article DOI: 10.1021/acsmedchemlett.7b00032 BindingDB Entry DOI: 10.7270/Q2S46V8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/epsilon (Rattus norvegicus-RAT) | BDBM50235306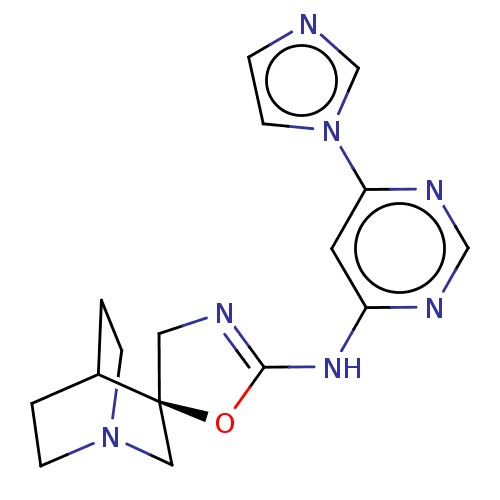 (CHEMBL4084621) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at rat alpha1beta1delta1epsilon nAChR expressed in HEK293 cells assessed as increase in Ca2+ flux by Fluo-4 AM dye based FLIPR assay | ACS Med Chem Lett 8: 366-371 (2017) Article DOI: 10.1021/acsmedchemlett.7b00032 BindingDB Entry DOI: 10.7270/Q2S46V8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/epsilon (Rattus norvegicus-RAT) | BDBM50206243 (CHEMBL3918431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at rat alpha1beta1deltaepsilon nAChR expressed in HEK293 cells for 2 mins by FLIPR assay | J Med Chem 59: 11171-11181 (2016) Article DOI: 10.1021/acs.jmedchem.6b01506 BindingDB Entry DOI: 10.7270/Q2NS0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||