 Found 3313 hits of affinity data for UniProtKB/TrEMBL: P11511
Found 3313 hits of affinity data for UniProtKB/TrEMBL: P11511 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM19461
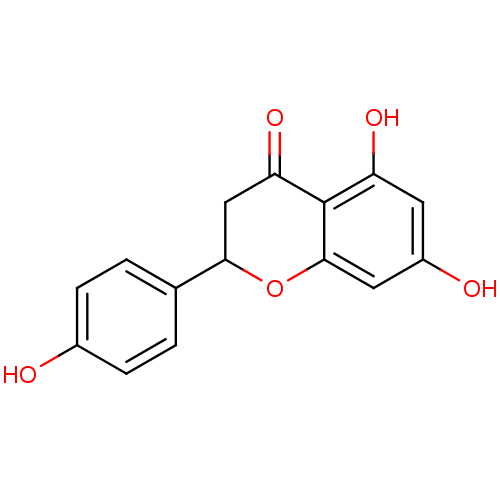
(α-CA inhibitor, 5 | 5,7-dihydroxy-2-(4-hydrox...)Show InChI InChI=1S/C15H12O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-6,13,16-18H,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00110 | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM92556
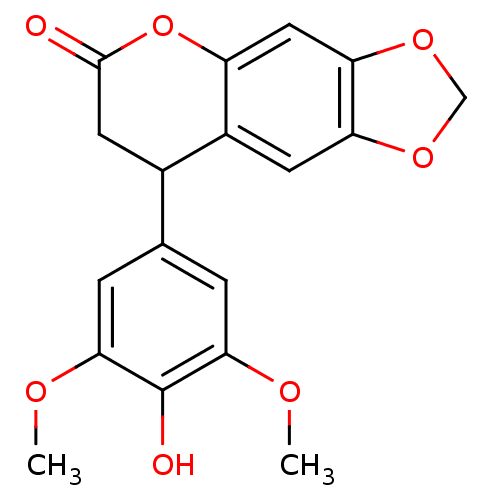
(Neoflavonoid, 8)Show InChI InChI=1S/C18H16O7/c1-21-15-3-9(4-16(22-2)18(15)20)10-6-17(19)25-12-7-14-13(5-11(10)12)23-8-24-14/h3-5,7,10,20H,6,8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00182 | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM92555
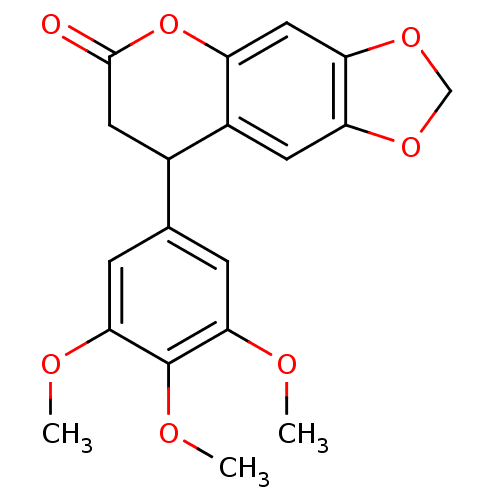
(Neoflavonoid, 7)Show InChI InChI=1S/C19H18O7/c1-21-16-4-10(5-17(22-2)19(16)23-3)11-7-18(20)26-13-8-15-14(6-12(11)13)24-9-25-15/h4-6,8,11H,7,9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM92557
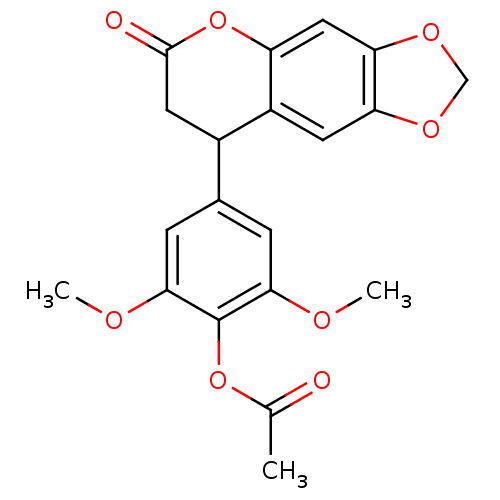
(Neoflavonoid, 9)Show SMILES COc1cc(cc(OC)c1OC(C)=O)C1CC(=O)Oc2cc3OCOc3cc12 Show InChI InChI=1S/C20H18O8/c1-10(21)27-20-17(23-2)4-11(5-18(20)24-3)12-7-19(22)28-14-8-16-15(6-13(12)14)25-9-26-16/h4-6,8,12H,7,9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM92559

(Neoflavonoid, 11)Show SMILES COc1cc(OC)c2C(CC(=O)Oc2c1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C20H22O7/c1-22-12-8-14(23-2)19-13(10-18(21)27-15(19)9-12)11-6-16(24-3)20(26-5)17(7-11)25-4/h6-9,13H,10H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM92560
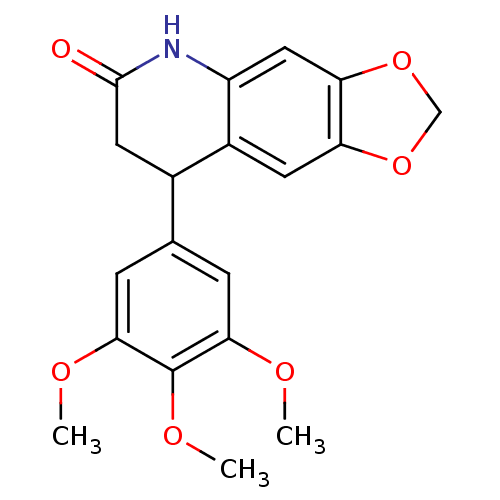
(Neoflavonoid, 19)Show InChI InChI=1S/C19H19NO6/c1-22-16-4-10(5-17(23-2)19(16)24-3)11-7-18(21)20-13-8-15-14(6-12(11)13)25-9-26-15/h4-6,8,11H,7,9H2,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00597 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM92558
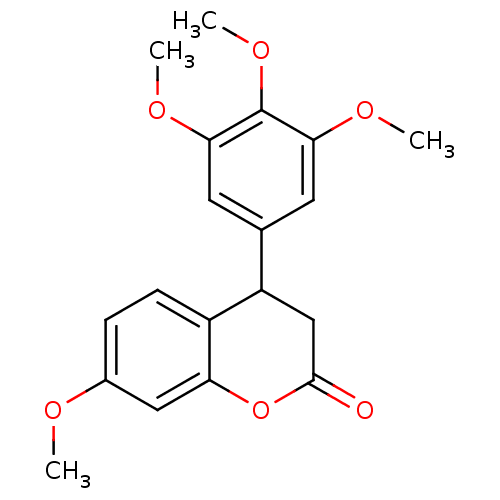
(Neoflavonoid, 10)Show InChI InChI=1S/C19H20O6/c1-21-12-5-6-13-14(10-18(20)25-15(13)9-12)11-7-16(22-2)19(24-4)17(8-11)23-3/h5-9,14H,10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00838 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM92561
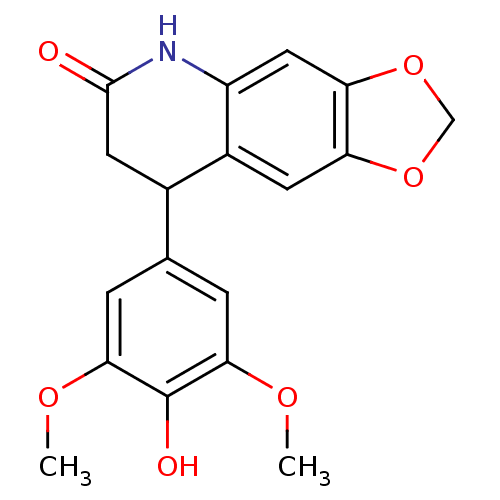
(Neoflavonoid, 20)Show InChI InChI=1S/C18H17NO6/c1-22-15-3-9(4-16(23-2)18(15)21)10-6-17(20)19-12-7-14-13(5-11(10)12)24-8-25-14/h3-5,7,10,21H,6,8H2,1-2H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Institute of Medicinal and Aromatic Plants
| Assay Description
Inhibition assay using aromatase enzyme. |
Chem Biol Drug Des 80: 616-624 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01439.x
BindingDB Entry DOI: 10.7270/Q24X56CG |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM13061

(4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...)Show InChI InChI=1S/C17H11N5/c18-9-13-1-5-15(6-2-13)17(22-12-20-11-21-22)16-7-3-14(10-19)4-8-16/h1-8,11-12,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... |
Bioorg Med Chem 20: 2427-34 (2012)
Article DOI: 10.1016/j.bmc.2012.01.047
BindingDB Entry DOI: 10.7270/Q2DJ5G3W |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... |
Bioorg Med Chem 20: 2427-34 (2012)
Article DOI: 10.1016/j.bmc.2012.01.047
BindingDB Entry DOI: 10.7270/Q2DJ5G3W |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50366125
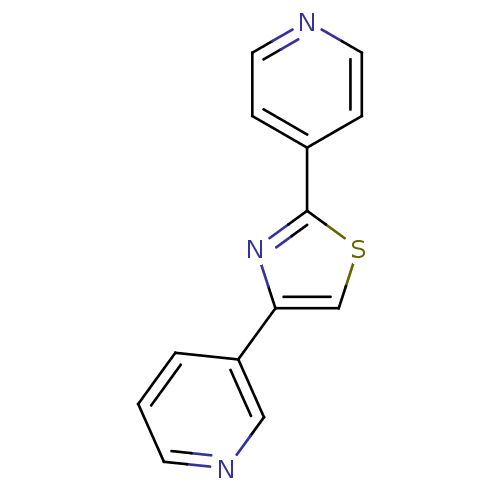
(CHEMBL1957214)Show InChI InChI=1S/C13H9N3S/c1-2-11(8-15-5-1)12-9-17-13(16-12)10-3-6-14-7-4-10/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... |
Bioorg Med Chem 20: 2427-34 (2012)
Article DOI: 10.1016/j.bmc.2012.01.047
BindingDB Entry DOI: 10.7270/Q2DJ5G3W |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10015
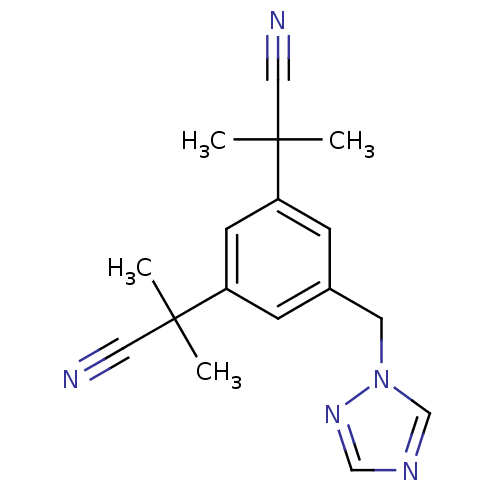
(2-[3-(1-cyano-1-methylethyl)-5-(1H-1,2,4-triazol-1...)Show InChI InChI=1S/C17H19N5/c1-16(2,9-18)14-5-13(8-22-12-20-11-21-22)6-15(7-14)17(3,4)10-19/h5-7,11-12H,8H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... |
Bioorg Med Chem 20: 2427-34 (2012)
Article DOI: 10.1016/j.bmc.2012.01.047
BindingDB Entry DOI: 10.7270/Q2DJ5G3W |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50240798
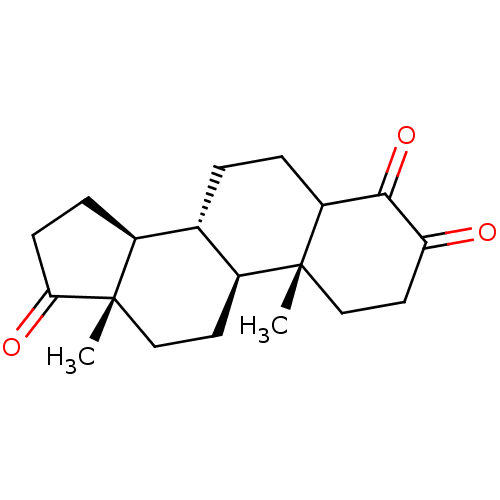
((8R,9S,10R,13S,14S)-4-Hydroxy-10,13-dimethyl-1,6,7...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4C(=O)C(=O)CC[C@]34C)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H26O3/c1-18-10-8-15(20)17(22)14(18)4-3-11-12-5-6-16(21)19(12,2)9-7-13(11)18/h11-14H,3-10H2,1-2H3/t11-,12-,13-,14?,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie
Curated by ChEMBL
| Assay Description
Inhibition constant for human aromatase cytochrome P450 19A1 activity |
Bioorg Med Chem Lett 8: 1041-4 (1999)
BindingDB Entry DOI: 10.7270/Q2445KM7 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50366128
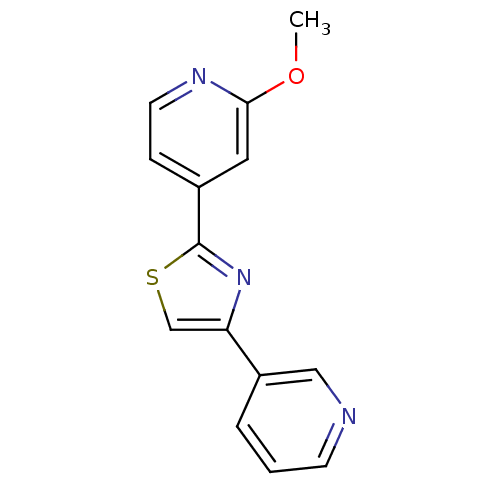
(CHEMBL1957217)Show InChI InChI=1S/C14H11N3OS/c1-18-13-7-10(4-6-16-13)14-17-12(9-19-14)11-3-2-5-15-8-11/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... |
Bioorg Med Chem 20: 2427-34 (2012)
Article DOI: 10.1016/j.bmc.2012.01.047
BindingDB Entry DOI: 10.7270/Q2DJ5G3W |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50398447
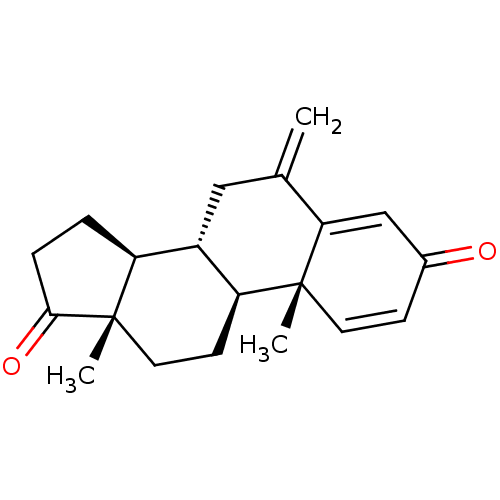
(Aromasin | EXEMESTANE)Show SMILES C[C@]12CC[C@H]3[C@@H](CC(=C)C4=CC(=O)C=C[C@]34C)[C@@H]1CCC2=O |r,c:13,t:9| Show InChI InChI=1S/C20H24O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h6,8,11,14-16H,1,4-5,7,9-10H2,2-3H3/t14-,15-,16-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM50014307
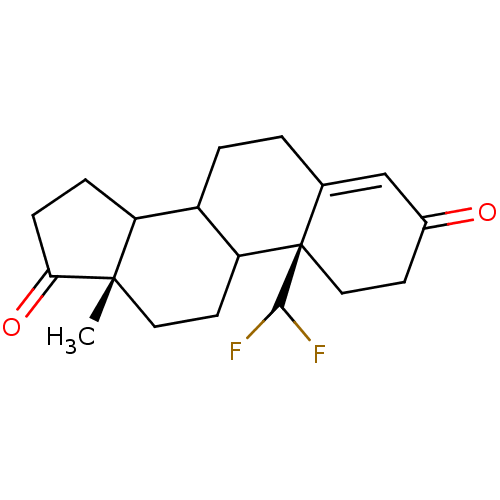
(10-Difluoromethyl-13-methyl-1,6,7,8,9,10,11,12,13,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C(F)F)C1CCC2=O |t:8| Show InChI InChI=1S/C19H24F2O2/c1-18-8-7-15-13(14(18)4-5-16(18)23)3-2-11-10-12(22)6-9-19(11,15)17(20)21/h10,13-15,17H,2-9H2,1H3/t13?,14?,15?,18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity was measured on Cytochrome P450 19A1 |
J Med Chem 33: 2933-42 (1990)
BindingDB Entry DOI: 10.7270/Q2VM4CW9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10044
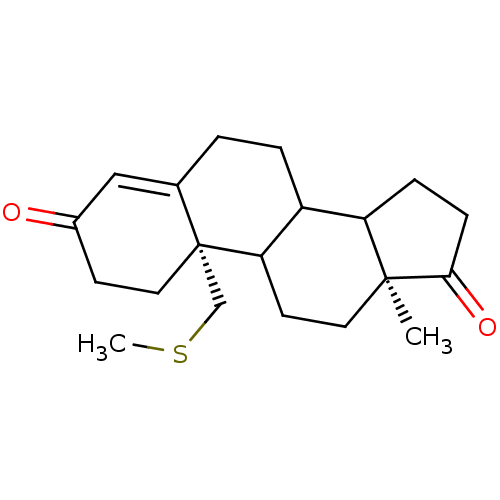
((2S,15S)-15-methyl-2-[(methylsulfanyl)methyl]tetra...)Show SMILES CSC[C@]12CCC(=O)C=C1CCC1C3CCC(=O)[C@@]3(C)CCC21 |r,c:8| Show InChI InChI=1S/C20H28O2S/c1-19-9-8-17-15(16(19)5-6-18(19)22)4-3-13-11-14(21)7-10-20(13,17)12-23-2/h11,15-17H,3-10,12H2,1-2H3/t15?,16?,17?,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity was measured on Cytochrome P450 19A1 |
J Med Chem 33: 2933-42 (1990)
BindingDB Entry DOI: 10.7270/Q2VM4CW9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50136071
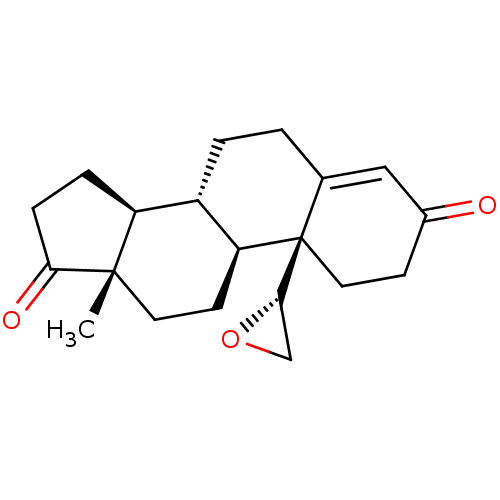
(CHEMBL3753593)Show SMILES [H][C@]1(CO1)[C@]12CCC(=O)C=C1CC[C@@]1([H])[C@]3([H])CCC(=O)[C@@]3(C)CC[C@]21[H] |r,c:10| Show InChI InChI=1S/C20H26O3/c1-19-8-7-16-14(15(19)4-5-17(19)22)3-2-12-10-13(21)6-9-20(12,16)18-11-23-18/h10,14-16,18H,2-9,11H2,1H3/t14-,15-,16-,18-,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10044

((2S,15S)-15-methyl-2-[(methylsulfanyl)methyl]tetra...)Show SMILES CSC[C@]12CCC(=O)C=C1CCC1C3CCC(=O)[C@@]3(C)CCC21 |r,c:8| Show InChI InChI=1S/C20H28O2S/c1-19-9-8-17-15(16(19)5-6-18(19)22)4-3-13-11-14(21)7-10-20(13,17)12-23-2/h11,15-17H,3-10,12H2,1-2H3/t15?,16?,17?,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Aromatase inhibitor potency as iron-binding-related type II difference spectrum |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011771
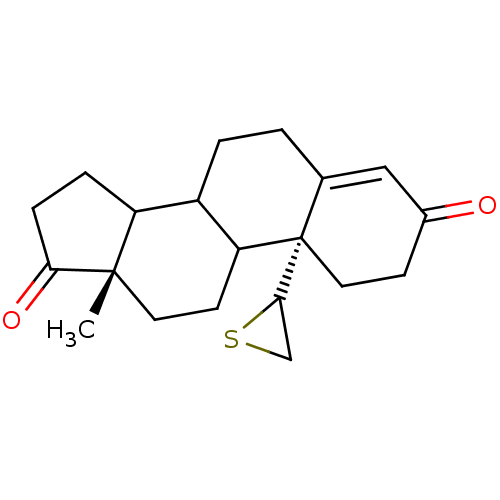
(13-Methyl-10-thiiranyl-1,6,7,8,9,10,11,12,13,14,15...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@@]34C3CS3)C1CCC2=O |t:8| Show InChI InChI=1S/C20H26O2S/c1-19-8-7-16-14(15(19)4-5-17(19)22)3-2-12-10-13(21)6-9-20(12,16)18-11-23-18/h10,14-16,18H,2-9,11H2,1H3/t14?,15?,16?,18?,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity was measured on Cytochrome P450 19A1 |
J Med Chem 33: 2933-42 (1990)
BindingDB Entry DOI: 10.7270/Q2VM4CW9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011771

(13-Methyl-10-thiiranyl-1,6,7,8,9,10,11,12,13,14,15...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@@]34C3CS3)C1CCC2=O |t:8| Show InChI InChI=1S/C20H26O2S/c1-19-8-7-16-14(15(19)4-5-17(19)22)3-2-12-10-13(21)6-9-20(12,16)18-11-23-18/h10,14-16,18H,2-9,11H2,1H3/t14?,15?,16?,18?,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human placental Cytochrome P450 19A1 |
J Med Chem 34: 1344-9 (1991)
BindingDB Entry DOI: 10.7270/Q2M044C9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50014316

(7-(4-Azido-phenylsulfanyl)-10,13-dimethyl-1,6,7,8,...)Show SMILES C[C@]12CCC3C(C1CCC2=O)[C@@H](CC1=CC(=O)CC[C@]31C)Sc1ccc(cc1)N=[N+]=[N-] |t:15| Show InChI InChI=1S/C25H29N3O2S/c1-24-11-9-17(29)13-15(24)14-21(31-18-5-3-16(4-6-18)27-28-26)23-19-7-8-22(30)25(19,2)12-10-20(23)24/h3-6,13,19-21,23H,7-12,14H2,1-2H3/t19?,20?,21-,23?,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity was measured on Cytochrome P450 19A1 |
J Med Chem 33: 2933-42 (1990)
BindingDB Entry DOI: 10.7270/Q2VM4CW9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50014316

(7-(4-Azido-phenylsulfanyl)-10,13-dimethyl-1,6,7,8,...)Show SMILES C[C@]12CCC3C(C1CCC2=O)[C@@H](CC1=CC(=O)CC[C@]31C)Sc1ccc(cc1)N=[N+]=[N-] |t:15| Show InChI InChI=1S/C25H29N3O2S/c1-24-11-9-17(29)13-15(24)14-21(31-18-5-3-16(4-6-18)27-28-26)23-19-7-8-22(30)25(19,2)12-10-20(23)24/h3-6,13,19-21,23H,7-12,14H2,1-2H3/t19?,20?,21-,23?,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity was measured on Cytochrome P450 19A1 |
J Med Chem 33: 2933-42 (1990)
BindingDB Entry DOI: 10.7270/Q2VM4CW9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135997

(CHEMBL3754471)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@H](C)C2=CC(=O)CC[C@]12C |r,t:19| Show InChI InChI=1S/C20H28O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h11-12,14-16H,4-10H2,1-3H3/t12-,14-,15-,16-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50041376

((R)-6-Ethyl-10,13-dimethyl-1,6,7,8,9,10,11,12,13,1...)Show SMILES CC[C@@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)CCC(=O)C=C12 |t:23| Show InChI InChI=1S/C21H30O2/c1-4-13-11-15-16-5-6-19(23)21(16,3)10-8-17(15)20(2)9-7-14(22)12-18(13)20/h12-13,15-17H,4-11H2,1-3H3/t13-,15?,16?,17?,20?,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Binding affinity for human placental microsome Cytochrome P450 19A1 |
J Med Chem 37: 1312-9 (1994)
BindingDB Entry DOI: 10.7270/Q2NZ86PN |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aromatase
(Homo sapiens (Human)) | BDBM50136063

(CHEMBL3752102)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@@H](CCCC)C2=CC(=O)CC[C@]12C |r,t:22| Show InChI InChI=1S/C23H34O2/c1-4-5-6-15-13-17-18-7-8-21(25)23(18,3)12-10-19(17)22(2)11-9-16(24)14-20(15)22/h14-15,17-19H,4-13H2,1-3H3/t15-,17+,18+,19+,22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135998

(CHEMBL3752668)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@H](CCCC)C2=CC(=O)CC[C@]12C |r,t:22| Show InChI InChI=1S/C23H34O2/c1-4-5-6-15-13-17-18-7-8-21(25)23(18,3)12-10-19(17)22(2)11-9-16(24)14-20(15)22/h14-15,17-19H,4-13H2,1-3H3/t15-,17-,18-,19-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50136000
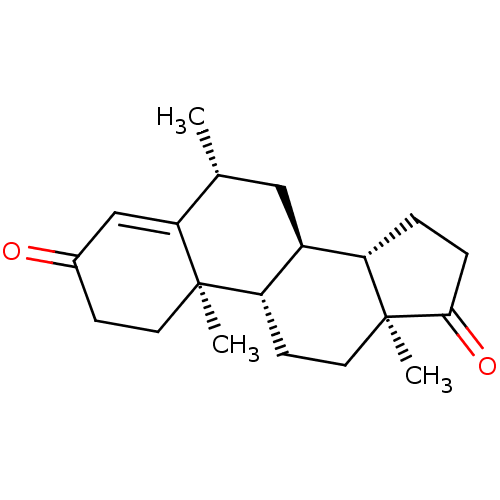
(CHEMBL3754220)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@@H](C)C2=CC(=O)CC[C@]12C |r,t:19| Show InChI InChI=1S/C20H28O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h11-12,14-16H,4-10H2,1-3H3/t12-,14+,15+,16+,19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50136057
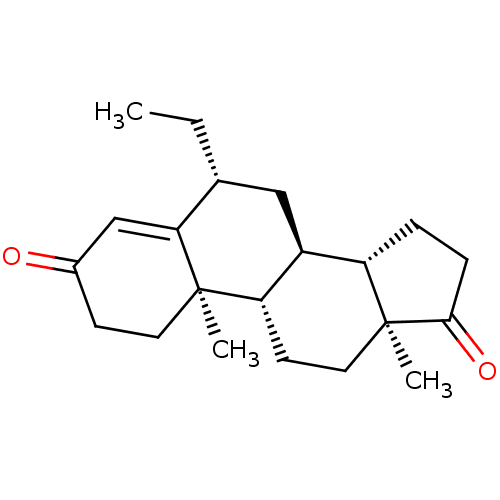
(CHEMBL3752661)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@@H](CC)C2=CC(=O)CC[C@]12C |r,t:20| Show InChI InChI=1S/C21H30O2/c1-4-13-11-15-16-5-6-19(23)21(16,3)10-8-17(15)20(2)9-7-14(22)12-18(13)20/h12-13,15-17H,4-11H2,1-3H3/t13-,15+,16+,17+,20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 10 mins in presence of [1beta-3H]androstenedione |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity was measured on Cytochrome P450 19A1 |
J Med Chem 33: 2933-42 (1990)
BindingDB Entry DOI: 10.7270/Q2VM4CW9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50405683

(CHEMBL174909)Show InChI InChI=1S/C15H18N2O2/c1-2-3-8-17-13(18)12-9-15(12,14(17)19)10-4-6-11(16)7-5-10/h4-7,12H,2-3,8-9,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Apparent inhibition constant (Ki) for cytochrome P450 19A1 with androstenedione |
J Med Chem 31: 971-6 (1988)
BindingDB Entry DOI: 10.7270/Q2154J7X |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114658
BindingDB Entry DOI: 10.7270/Q2NP28CD |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50254017

(6-((4-fluorophenyl)(1H-imidazol-1-yl)methyl)benzo[...)Show InChI InChI=1S/C17H13FN2O3/c18-12-3-1-11(2-4-12)17(20-6-5-19-9-20)13-7-15-16(8-14(13)21)23-10-22-15/h1-9,17,21H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by Lineweaver-Burk plot in presence of NADPH |
J Med Chem 52: 143-50 (2009)
Article DOI: 10.1021/jm800945c
BindingDB Entry DOI: 10.7270/Q2SQ9080 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM13061

(4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...)Show InChI InChI=1S/C17H11N5/c18-9-13-1-5-15(6-2-13)17(22-12-20-11-21-22)16-7-3-14(10-19)4-8-16/h1-8,11-12,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra
Curated by ChEMBL
| Assay Description
Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by Lineweaver-Burk plot in presence of NADPH |
J Med Chem 52: 143-50 (2009)
Article DOI: 10.1021/jm800945c
BindingDB Entry DOI: 10.7270/Q2SQ9080 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50014299
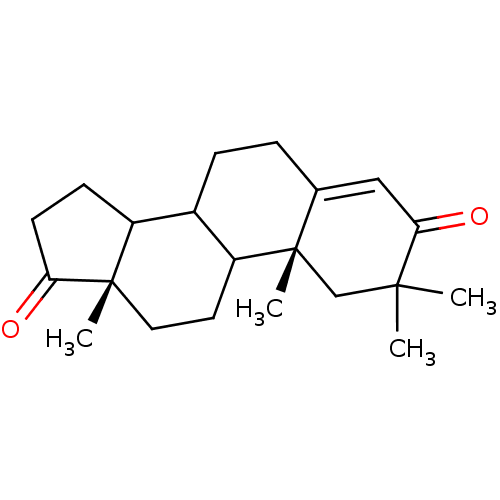
(2,2,10,13-Tetramethyl-1,6,7,8,9,10,11,12,13,14,15,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)C(C)(C)C[C@]34C)C1CCC2=O |t:8| Show InChI InChI=1S/C21H30O2/c1-19(2)12-21(4)13(11-18(19)23)5-6-14-15-7-8-17(22)20(15,3)10-9-16(14)21/h11,14-16H,5-10,12H2,1-4H3/t14?,15?,16?,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity was measured on Cytochrome P450 19A1 |
J Med Chem 33: 2933-42 (1990)
BindingDB Entry DOI: 10.7270/Q2VM4CW9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aromatase
(Homo sapiens (Human)) | BDBM50135918
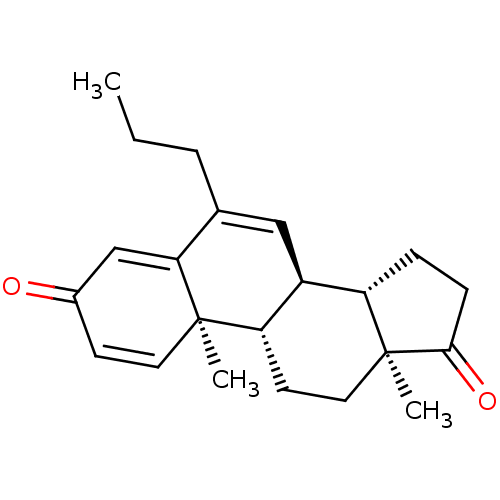
(CHEMBL3753803)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=C(CCC)C2=CC(=O)C=C[C@]12C |r,c:25,t:16,21| Show InChI InChI=1S/C22H28O2/c1-4-5-14-12-16-17-6-7-20(24)22(17,3)11-9-18(16)21(2)10-8-15(23)13-19(14)21/h8,10,12-13,16-18H,4-7,9,11H2,1-3H3/t16-,17-,18-,21+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135921

(CHEMBL3752011)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=C(CCCC)C2=CC(=O)C=C[C@]12C |r,c:26,t:16,22| Show InChI InChI=1S/C23H30O2/c1-4-5-6-15-13-17-18-7-8-21(25)23(18,3)12-10-19(17)22(2)11-9-16(24)14-20(15)22/h9,11,13-14,17-19H,4-8,10,12H2,1-3H3/t17-,18-,19-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
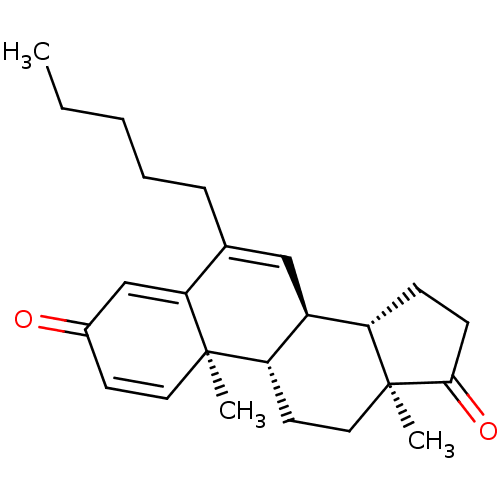
Aromatase
(Homo sapiens (Human)) | BDBM50135922
(CHEMBL3752619)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=C(CCCCC)C2=CC(=O)C=C[C@]12C |r,c:27,t:16,23| Show InChI InChI=1S/C24H32O2/c1-4-5-6-7-16-14-18-19-8-9-22(26)24(19,3)13-11-20(18)23(2)12-10-17(25)15-21(16)23/h10,12,14-15,18-20H,4-9,11,13H2,1-3H3/t18-,19-,20-,23+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135969

(CHEMBL3752315)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=C(CCCCCC)C2=CC(=O)C=C[C@]12C |r,c:28,t:16,24| Show InChI InChI=1S/C25H34O2/c1-4-5-6-7-8-17-15-19-20-9-10-23(27)25(20,3)14-12-21(19)24(2)13-11-18(26)16-22(17)24/h11,13,15-16,19-21H,4-10,12,14H2,1-3H3/t19-,20-,21-,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
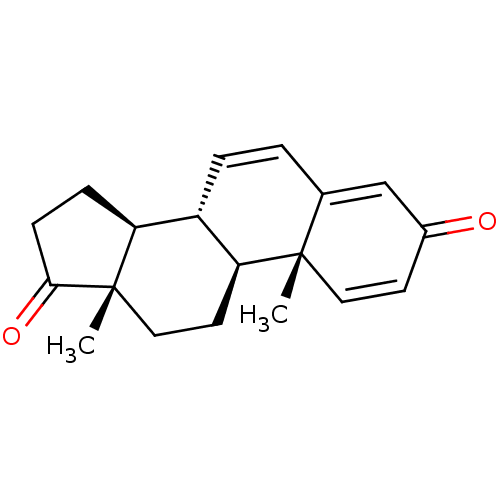
Aromatase
(Homo sapiens (Human)) | BDBM50421878
(CHEMBL2311178)Show SMILES C[C@]12CC[C@H]3[C@@H](C=CC4=CC(=O)C=C[C@]34C)[C@@H]1CCC2=O |c:6,12,t:8| Show InChI InChI=1S/C19H22O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h3-4,7,9,11,14-16H,5-6,8,10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135917

(CHEMBL3754546)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=C(CC)C2=CC(=O)C=C[C@]12C |r,c:24,t:16,20| Show InChI InChI=1S/C21H26O2/c1-4-13-11-15-16-5-6-19(23)21(16,3)10-8-17(15)20(2)9-7-14(22)12-18(13)20/h7,9,11-12,15-17H,4-6,8,10H2,1-3H3/t15-,16-,17-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135916

(CHEMBL3752165)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=C(C)C2=CC(=O)C=C[C@]12C |r,c:23,t:16,19| Show InChI InChI=1S/C20H24O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h6,8,10-11,14-16H,4-5,7,9H2,1-3H3/t14-,15-,16-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
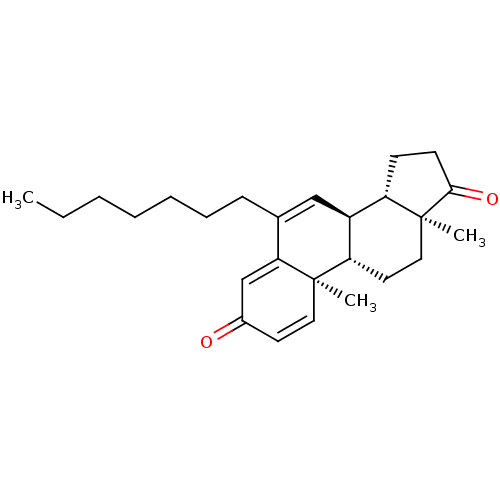
Aromatase
(Homo sapiens (Human)) | BDBM50135970
(CHEMBL3752341)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=C(CCCCCCC)C2=CC(=O)C=C[C@]12C |r,c:29,t:16,25| Show InChI InChI=1S/C26H36O2/c1-4-5-6-7-8-9-18-16-20-21-10-11-24(28)26(21,3)15-13-22(20)25(2)14-12-19(27)17-23(18)25/h12,14,16-17,20-22H,4-11,13,15H2,1-3H3/t20-,21-,22-,25+,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135799

(CHEMBL3752650)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@@H](CCCCC)C2=CC(=O)CC[C@]12C |r,t:23| Show InChI InChI=1S/C24H36O2/c1-4-5-6-7-16-14-18-19-8-9-22(26)24(19,3)13-11-20(18)23(2)12-10-17(25)15-21(16)23/h15-16,18-20H,4-14H2,1-3H3/t16-,18+,19+,20+,23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135857

(CHEMBL3754285)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@@H](CCCCCC)C2=CC(=O)CC[C@]12C |r,t:24| Show InChI InChI=1S/C25H38O2/c1-4-5-6-7-8-17-15-19-20-9-10-23(27)25(20,3)14-12-21(19)24(2)13-11-18(26)16-22(17)24/h16-17,19-21H,4-15H2,1-3H3/t17-,19+,20+,21+,24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50135858

(CHEMBL3754366)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@@H](CCCCCCCC)C2=CC(=O)CC[C@]12C |r,t:26| Show InChI InChI=1S/C27H42O2/c1-4-5-6-7-8-9-10-19-17-21-22-11-12-25(29)27(22,3)16-14-23(21)26(2)15-13-20(28)18-24(19)26/h18-19,21-23H,4-17H2,1-3H3/t19-,21+,22+,23+,26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The M. S. University of Baroda
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]AD |
Eur J Med Chem 105: 1-38 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.038
BindingDB Entry DOI: 10.7270/Q2W66NMZ |
More data for this
Ligand-Target Pair | |
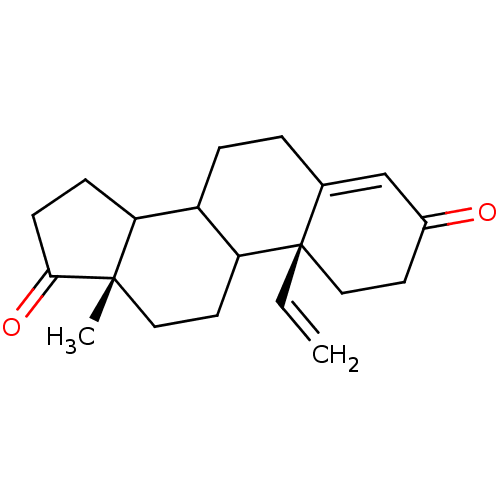
Aromatase
(Homo sapiens (Human)) | BDBM50014310
(13-Methyl-10-vinyl-1,6,7,8,9,10,11,12,13,14,15,16-...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C=C)C1CCC2=O |t:8| Show InChI InChI=1S/C20H26O2/c1-3-20-11-8-14(21)12-13(20)4-5-15-16-6-7-18(22)19(16,2)10-9-17(15)20/h3,12,15-17H,1,4-11H2,2H3/t15?,16?,17?,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity was measured on Cytochrome P450 19A1 |
J Med Chem 33: 2933-42 (1990)
BindingDB Entry DOI: 10.7270/Q2VM4CW9 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9955
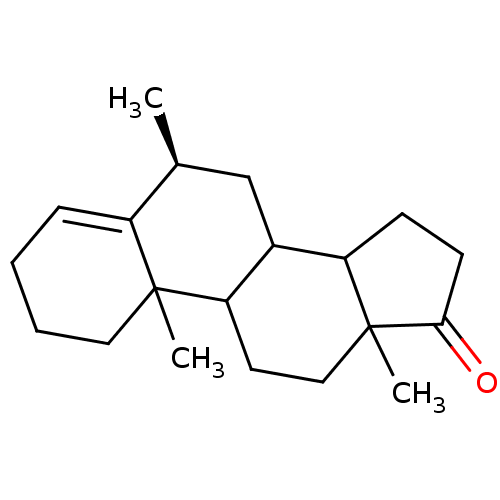
((8S)-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{1...)Show SMILES C[C@H]1CC2C3CCC(=O)C3(C)CCC2C2(C)CCCC=C12 |r,t:21| Show InChI InChI=1S/C20H30O/c1-13-12-14-16-7-8-18(21)20(16,3)11-9-17(14)19(2)10-5-4-6-15(13)19/h6,13-14,16-17H,4-5,7-12H2,1-3H3/t13-,14?,16?,17?,19?,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | -50.5 | 37 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy
| Assay Description
The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... |
J Med Chem 39: 2245-52 (1996)
Article DOI: 10.1021/jm960047o
BindingDB Entry DOI: 10.7270/Q2X34VPV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data