

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169033 (CHEMBL3806069) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169038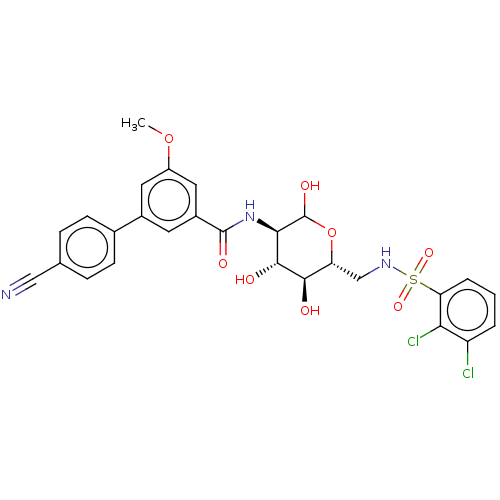 (CHEMBL3804841) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169026 (CHEMBL3805148) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169032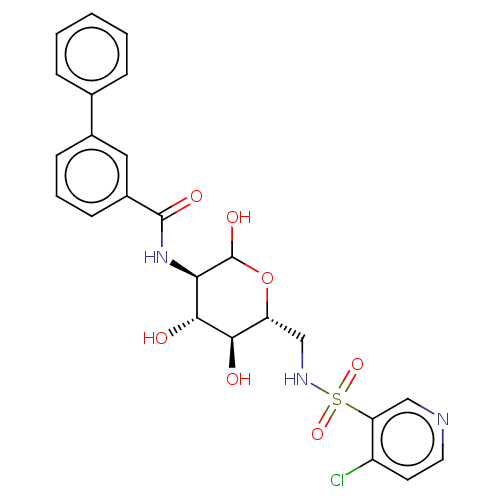 (CHEMBL3805398) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169037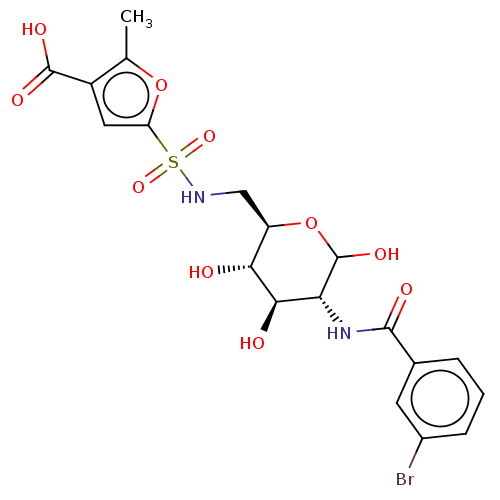 (CHEMBL3805205) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169034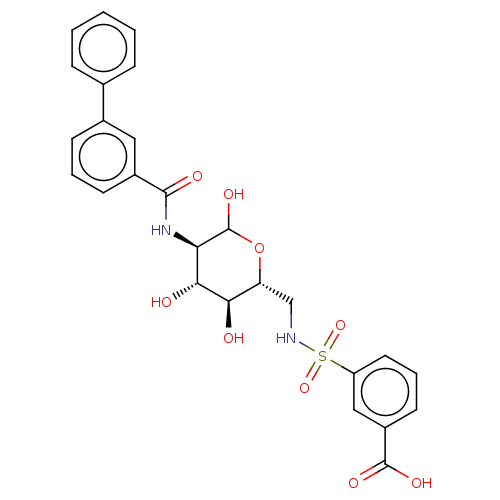 (CHEMBL3805734) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169031 (CHEMBL3805905) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169017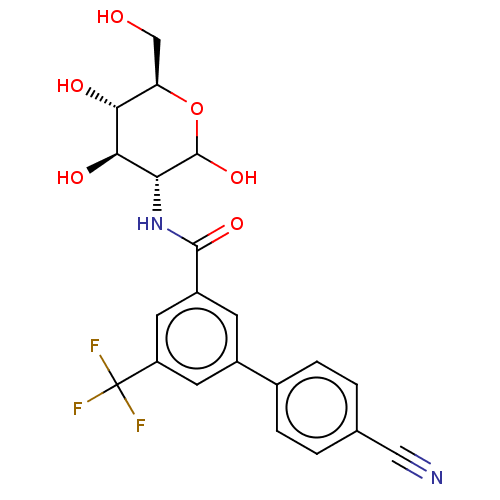 (CHEMBL3804930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169028 (CHEMBL3805598) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169023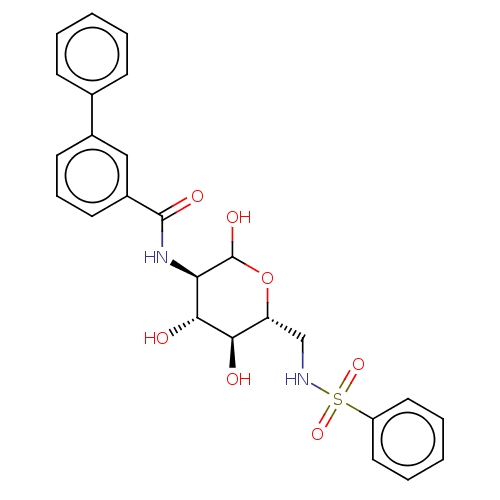 (CHEMBL3805753) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169015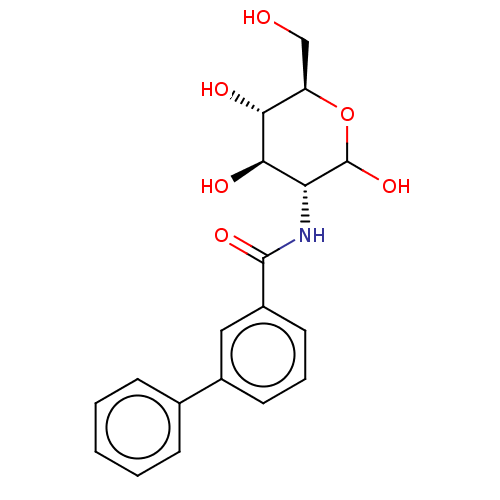 (CHEMBL3805460) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169042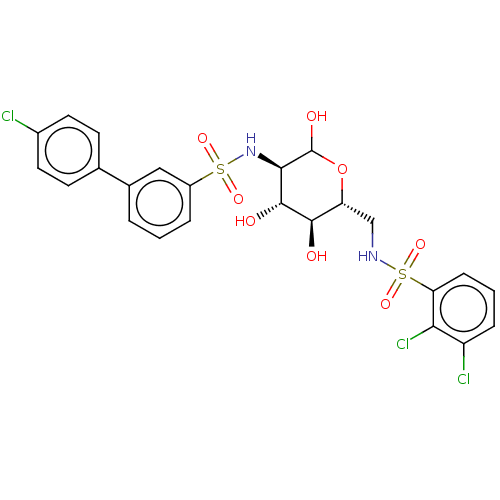 (CHEMBL3806103) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169036 (CHEMBL3806028) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169039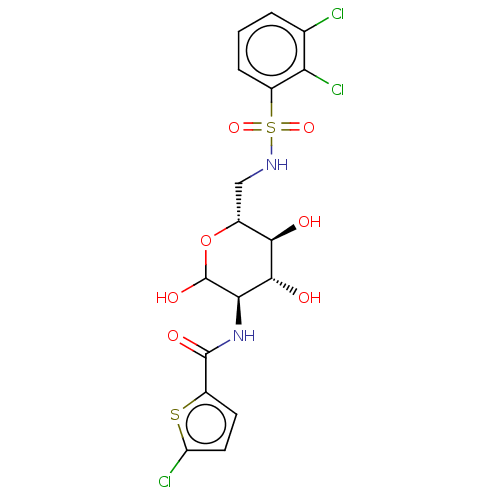 (CHEMBL3806132) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169019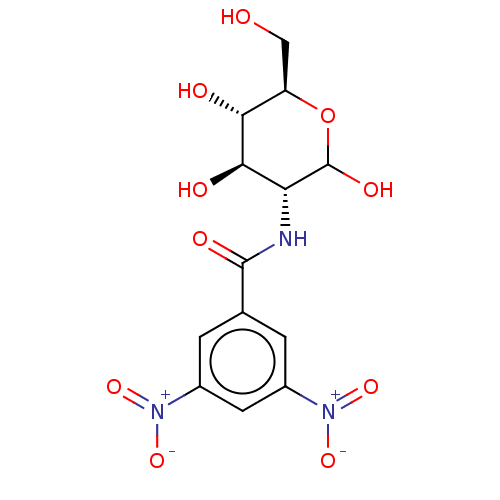 (CHEMBL3805765) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169013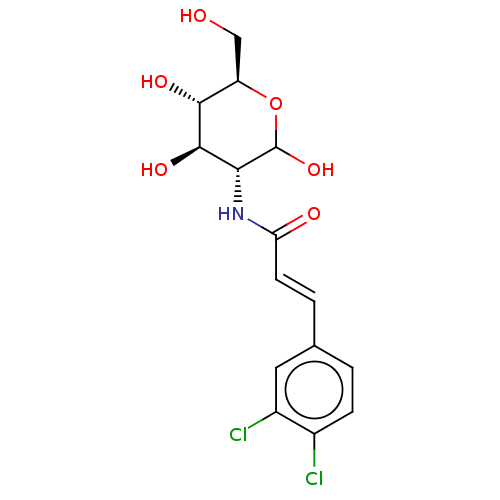 (CHEMBL3805703) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169025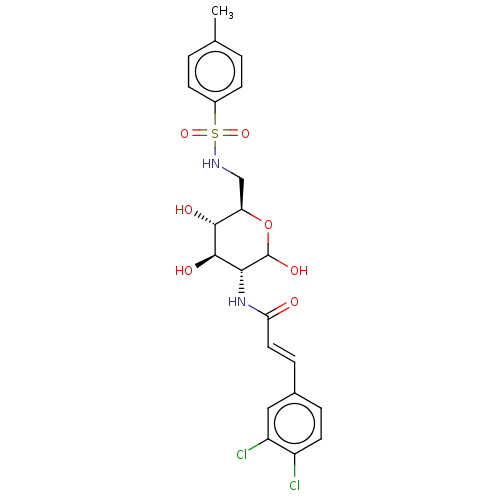 (CHEMBL3806250) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169041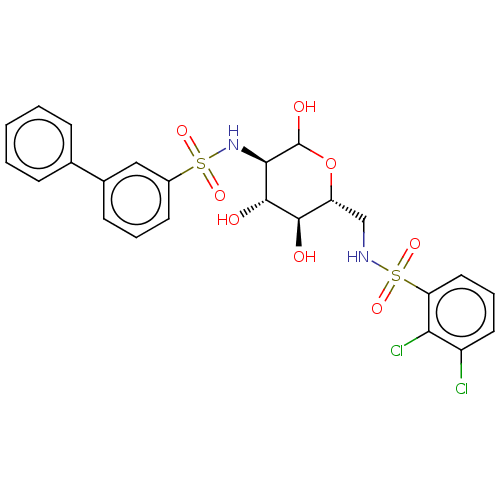 (CHEMBL3805653) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169040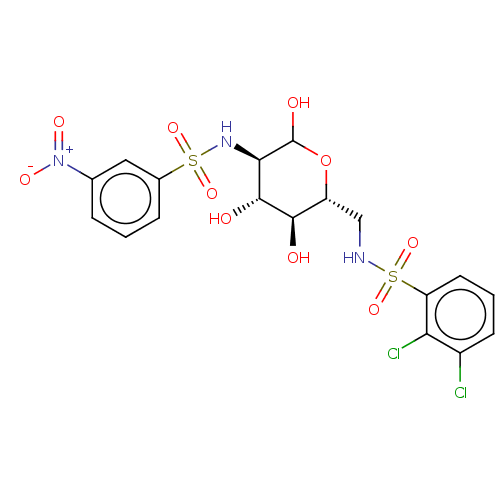 (CHEMBL3804874) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169014 (CHEMBL3806183) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169043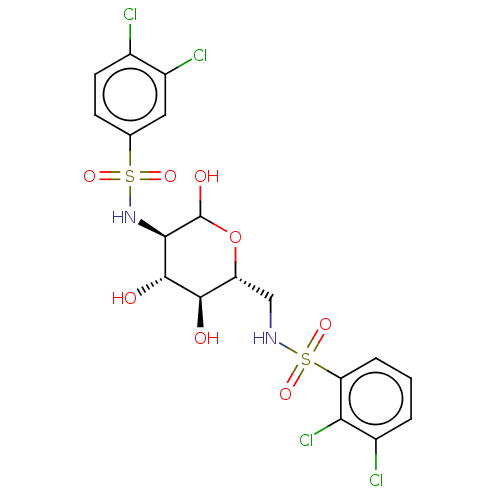 (CHEMBL3806095) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169046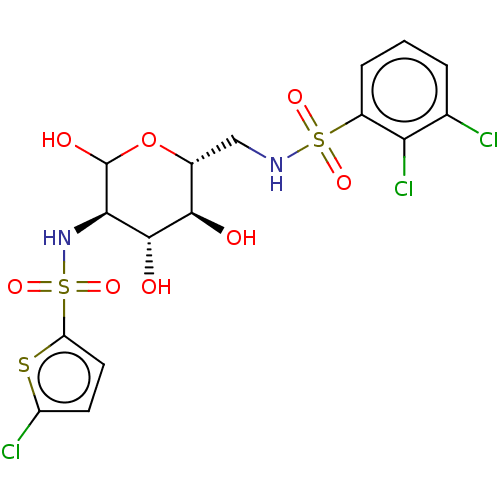 (CHEMBL3805459) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169018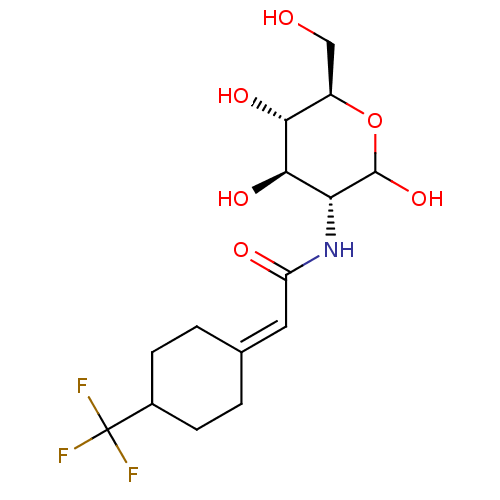 (CHEMBL3804924) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169020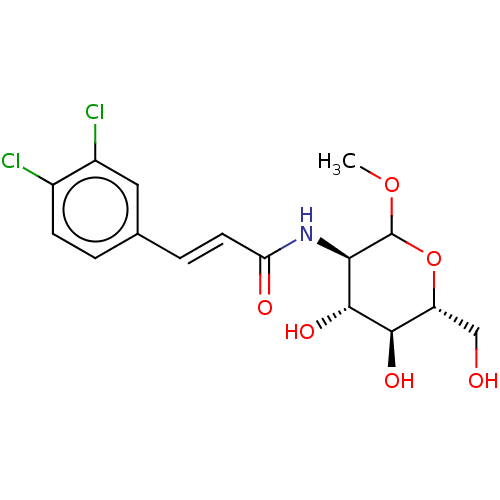 (CHEMBL3805806) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM50169022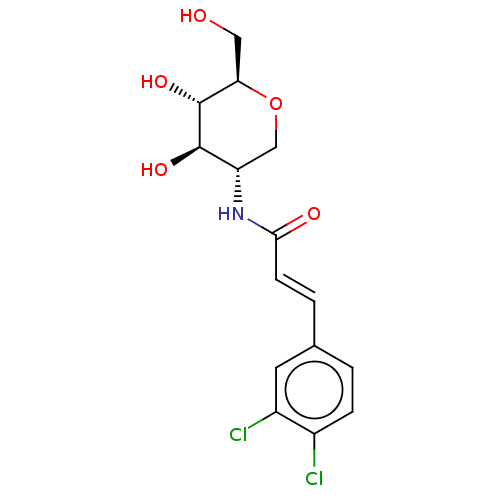 (CHEMBL3805752) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a... | ACS Med Chem Lett 7: 217-22 (2016) Article DOI: 10.1021/acsmedchemlett.5b00214 BindingDB Entry DOI: 10.7270/Q2PR7XWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM32004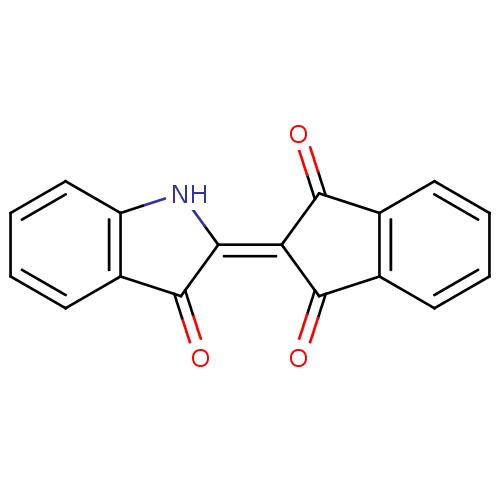 (2-(3-Oxo-1,3-dihydro-indol-2-ylidene)-indan-1,3-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM80764 (MLS000031522 | N-[2-[4-(2-hydroxyethyl)-1-piperazi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM80765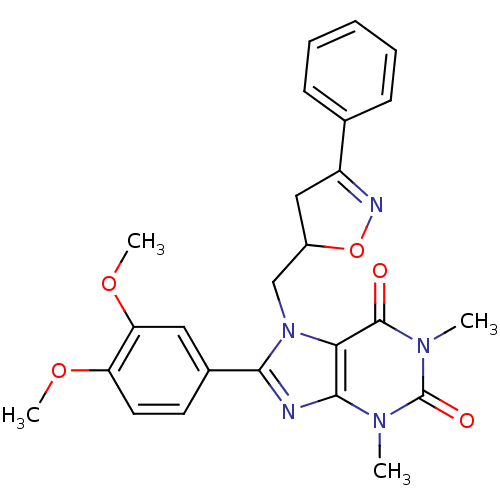 (8-(3,4-dimethoxyphenyl)-1,3-dimethyl-7-[(3-phenyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | n/a | n/a | 3.51E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM80766 (2-(7-fluoro-2-methyl-4-thiazolo[5,4-b]indolyl)acet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM34343 (4-(6-ethylindolo[2,3-b]quinoxalin-2-yl)sulfonylmor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM80767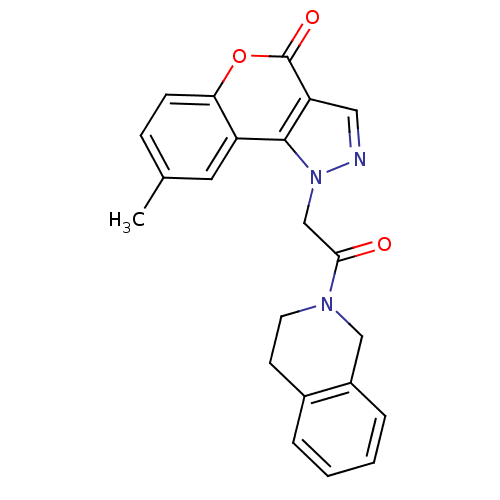 (1-[2-(3,4-dihydro-1H-isoquinolin-2-yl)-2-keto-ethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 3.67E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM80768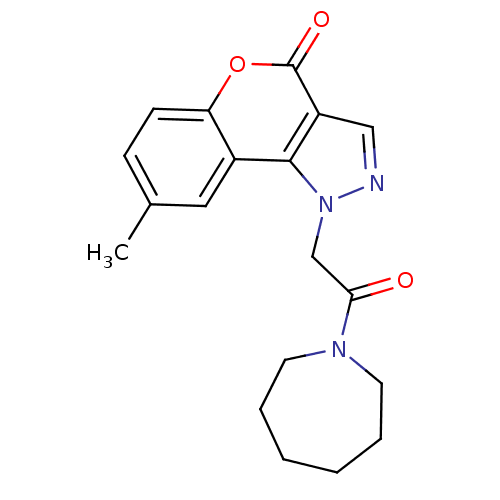 (1-[2-(1-azepanyl)-2-oxoethyl]-8-methyl-4-[1]benzop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 2.17E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM42405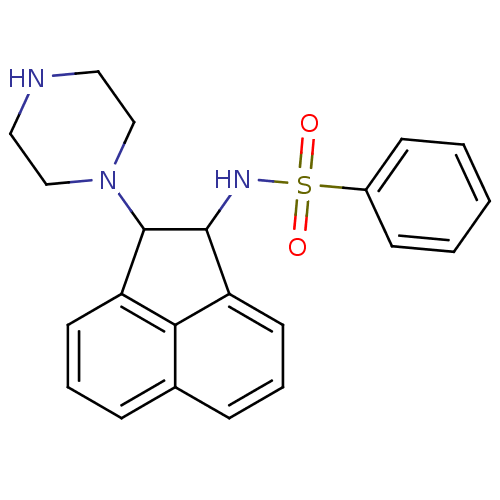 (MLS000110376 | N-(2-piperazin-1-yl-1,2-dihydroacen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM34648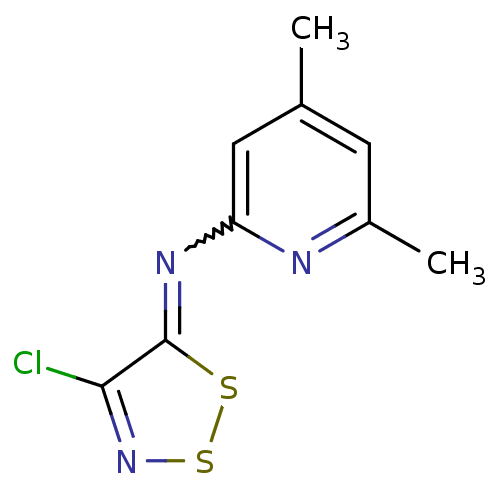 ((4-chlorodithiazol-5-ylidene)-(4,6-dimethyl-2-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | n/a | n/a | 3.99E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM80769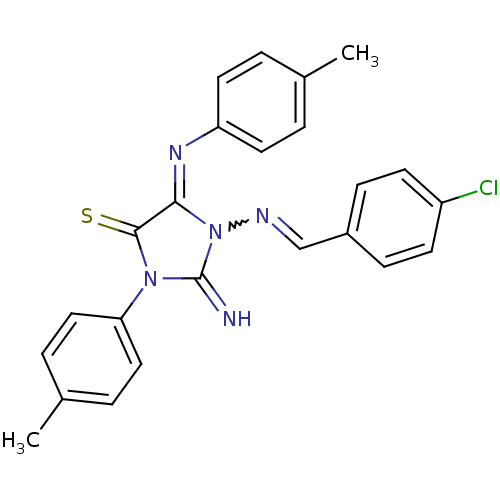 (1-[(4-chlorobenzylidene)amino]-2-imino-3-(4-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM80770 (4-(3-bromophenyl)-1,3-thiazol-2-amine | 4-(3-bromo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 4.93E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM48647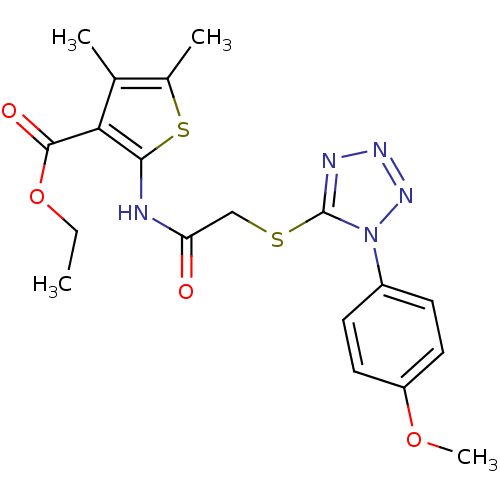 (2-[[2-[[1-(4-methoxyphenyl)-5-tetrazolyl]thio]-1-o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | n/a | n/a | 3.74E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM46685 ((4-chlorodithiazol-5-ylidene)-(p-tolyl)amine | 4-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 5.32E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM80773 ((3-chlorophenyl)-[5-(2,3-dihydro-1,4-benzodioxin-6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 4.13E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM48288 (MLS000565792 | N-ethyl-5-(4-methylphenyl)-6H-1,3,4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 6.31E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM80774 (5-(2,3-dihydro-1,4-benzodioxin-6-yl)-N-(4-methoxyp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 2.39E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM80775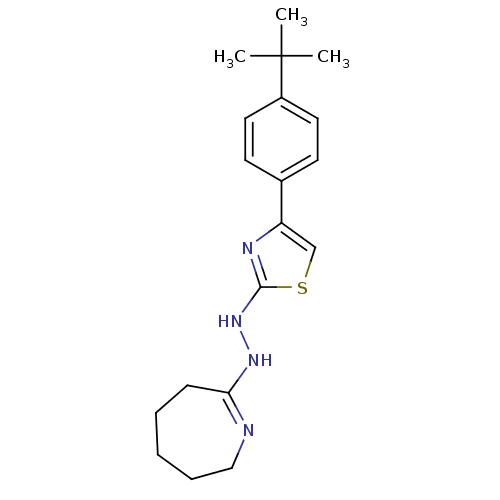 (1-[4-(4-tert-butylphenyl)-1,3-thiazol-2-yl]-2-(3,4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 4.74E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM74476 (4-({[1-(4-chlorophenyl)-3-methyl-5-oxo-1,5-dihydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM80776 (2-Acetylamino-5-benzyl-4-methyl-thiophene-3-carbox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 3.94E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM80777 ((6E)-6-[[(4-amino-1,2,4-triazol-3-yl)hydrazo]methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | n/a | n/a | 4.23E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM65111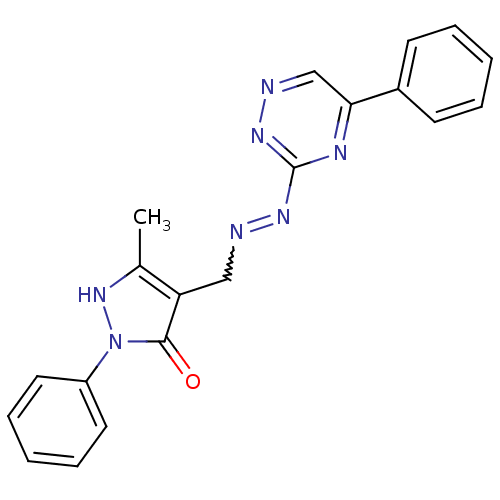 ((4E)-5-methyl-2-phenyl-4-[[(5-phenyl-1,2,4-triazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM67197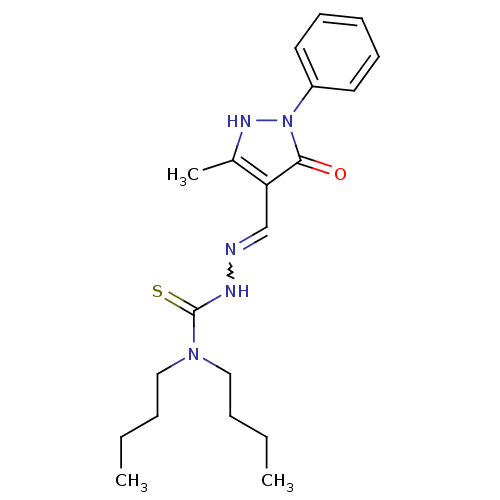 (1,1-dibutyl-3-[[(Z)-(3-methyl-5-oxidanylidene-1-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM80778 (3-(2-ketochromen-3-yl)-N-(4-pyrrolidinophenyl)benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM47735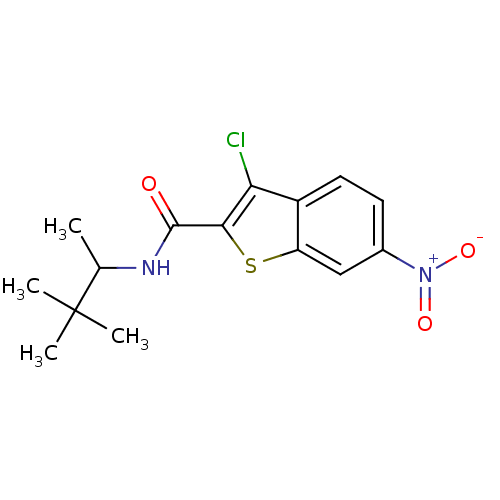 (3-chloranyl-N-(3,3-dimethylbutan-2-yl)-6-nitro-1-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 4.68E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-1 (Homo sapiens (Human)) | BDBM80779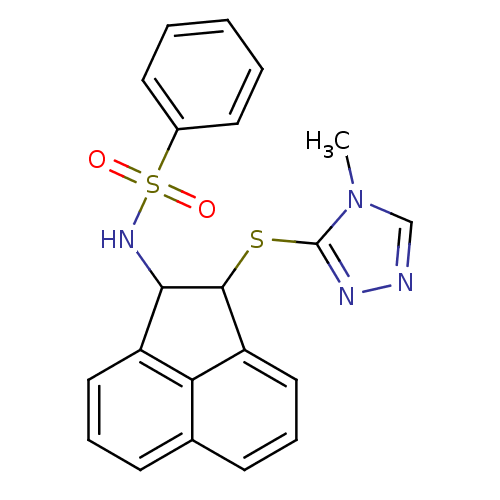 (MLS000547762 | N-[2-(4-Methyl-4H-[1,2,4]triazol-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 2.47E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2765CSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 126 total ) | Next | Last >> |