 Found 1432 hits for UniProtKB: P31751
Found 1432 hits for UniProtKB: P31751 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278836
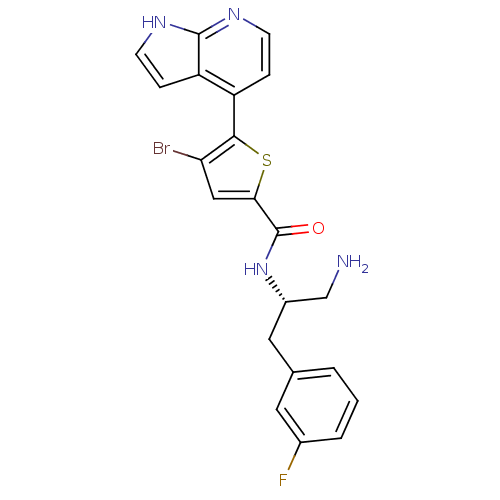
(CHEMBL523586 | N-((S)-1-amino-3-(3-fluorophenyl)pr...)Show SMILES NC[C@H](Cc1cccc(F)c1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H18BrFN4OS/c22-17-10-18(29-19(17)15-4-6-25-20-16(15)5-7-26-20)21(28)27-14(11-24)9-12-2-1-3-13(23)8-12/h1-8,10,14H,9,11,24H2,(H,25,26)(H,27,28)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316184

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316183
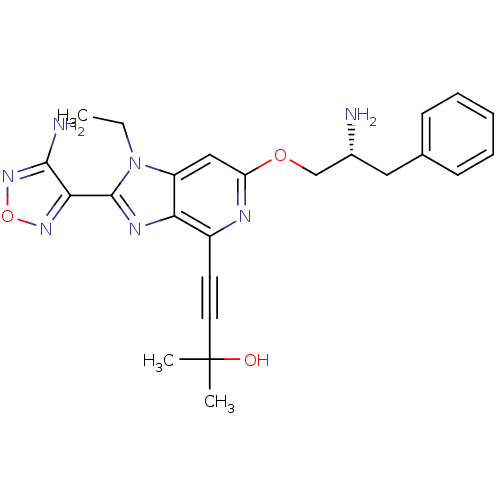
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(OC[C@H](N)Cc3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-18-13-19(33-14-16(25)12-15-8-6-5-7-9-15)27-17(10-11-24(2,3)32)20(18)28-23(31)21-22(26)30-34-29-21/h5-9,13,16,32H,4,12,14,25H2,1-3H3,(H2,26,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278837
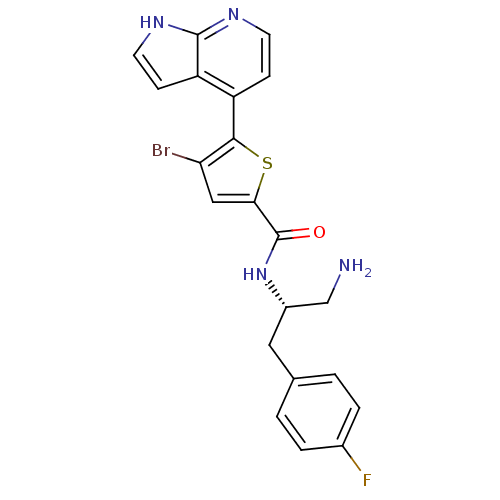
(CHEMBL496690 | N-((S)-1-amino-3-(4-fluorophenyl)pr...)Show SMILES NC[C@H](Cc1ccc(F)cc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H18BrFN4OS/c22-17-10-18(29-19(17)15-5-7-25-20-16(15)6-8-26-20)21(28)27-14(11-24)9-12-1-3-13(23)4-2-12/h1-8,10,14H,9,11,24H2,(H,25,26)(H,27,28)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278770

(CHEMBL470597 | N-((S)-1-amino-3-phenylpropan-2-yl)...)Show SMILES NC[C@H](Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278770

(CHEMBL470597 | N-((S)-1-amino-3-phenylpropan-2-yl)...)Show SMILES NC[C@H](Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278099
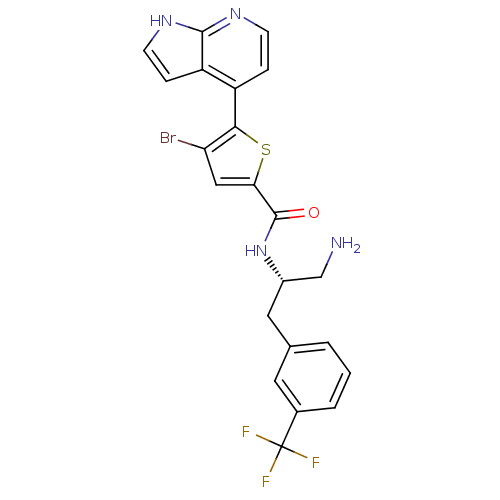
(CHEMBL482536 | N-((S)-1-amino-3-(3-(trifluoromethy...)Show SMILES NC[C@H](Cc1cccc(c1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-4-6-28-20-16(15)5-7-29-20)21(31)30-14(11-27)9-12-2-1-3-13(8-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278098
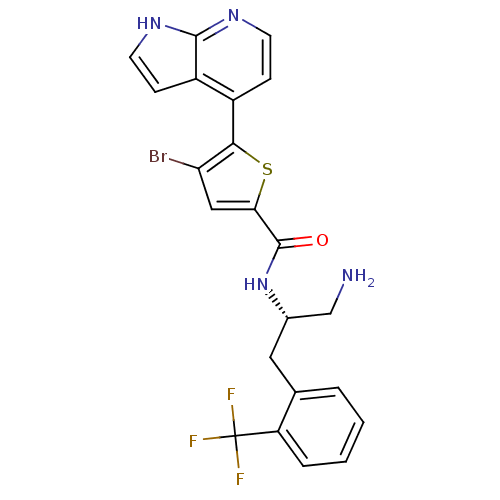
(CHEMBL520788 | N-((S)-1-amino-3-(2-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccccc1C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)14-5-7-28-20-15(14)6-8-29-20)21(31)30-13(11-27)9-12-3-1-2-4-16(12)22(24,25)26/h1-8,10,13H,9,11,27H2,(H,28,29)(H,30,31)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278098

(CHEMBL520788 | N-((S)-1-amino-3-(2-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccccc1C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)14-5-7-28-20-15(14)6-8-29-20)21(31)30-13(11-27)9-12-3-1-2-4-16(12)22(24,25)26/h1-8,10,13H,9,11,27H2,(H,28,29)(H,30,31)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25013
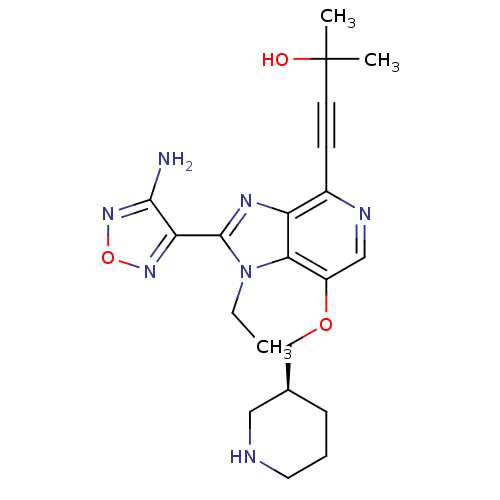
(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...)Show SMILES CCn1c(nc2c(ncc(OC[C@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316192
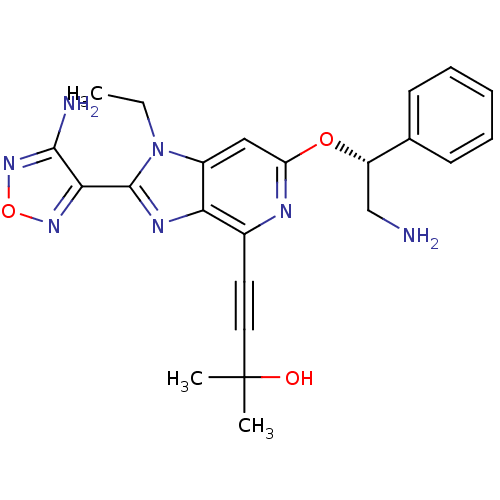
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278771
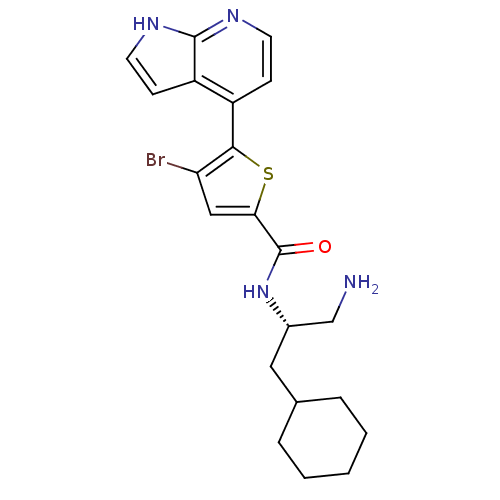
(CHEMBL470598 | N-((S)-1-amino-3-cyclohexylpropan-2...)Show SMILES NC[C@H](CC1CCCCC1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H25BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h6-9,11,13-14H,1-5,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278771

(CHEMBL470598 | N-((S)-1-amino-3-cyclohexylpropan-2...)Show SMILES NC[C@H](CC1CCCCC1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H25BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h6-9,11,13-14H,1-5,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278693
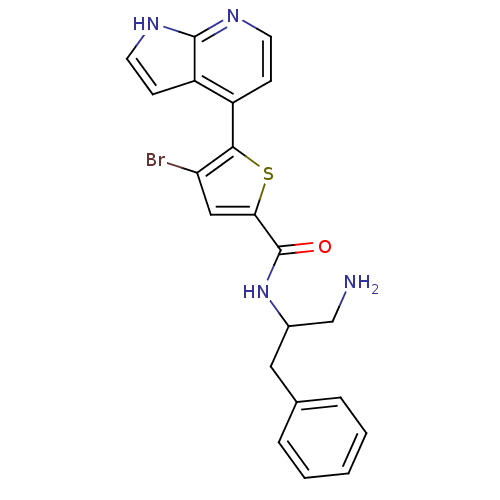
((+/-)-N-(1-amino-3-phenylpropan-2-yl)-4-bromo-5-(1...)Show SMILES NCC(Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278100
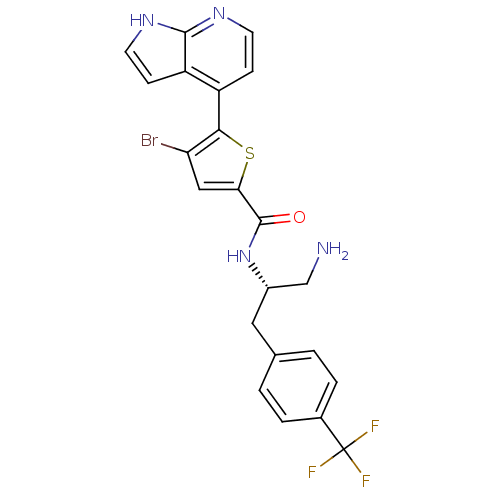
(CHEMBL482537 | N-((S)-1-amino-3-(4-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccc(cc1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-5-7-28-20-16(15)6-8-29-20)21(31)30-14(11-27)9-12-1-3-13(4-2-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278100

(CHEMBL482537 | N-((S)-1-amino-3-(4-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccc(cc1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-5-7-28-20-16(15)6-8-29-20)21(31)30-14(11-27)9-12-1-3-13(4-2-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316182
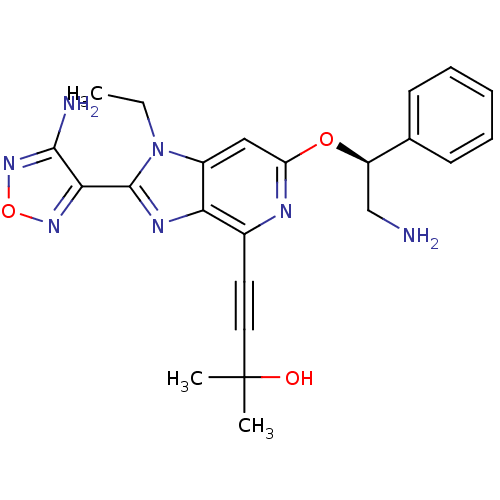
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316189
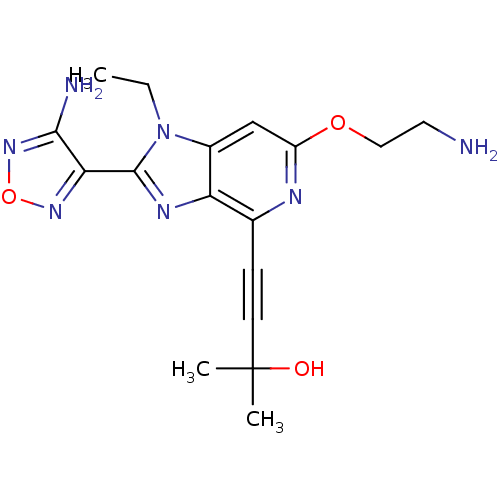
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-(2-aminoetho...)Show SMILES CCn1c(nc2c(nc(OCCN)cc12)C#CC(C)(C)O)-c1nonc1N Show InChI InChI=1S/C17H21N7O3/c1-4-24-11-9-12(26-8-7-18)20-10(5-6-17(2,3)25)13(11)21-16(24)14-15(19)23-27-22-14/h9,25H,4,7-8,18H2,1-3H3,(H2,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278834
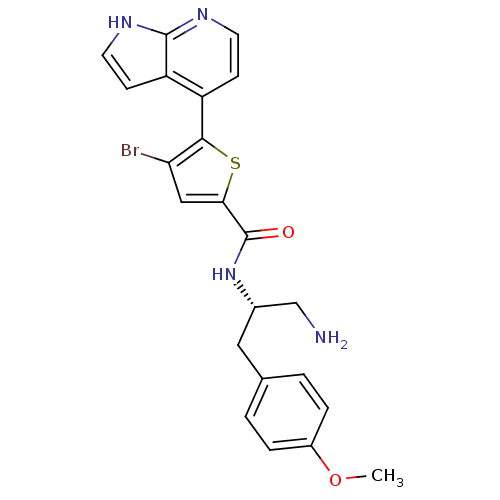
(CHEMBL524998 | N-((S)-1-amino-3-(4-methoxyphenyl)p...)Show SMILES COc1ccc(C[C@@H](CN)NC(=O)c2cc(Br)c(s2)-c2ccnc3[nH]ccc23)cc1 |r| Show InChI InChI=1S/C22H21BrN4O2S/c1-29-15-4-2-13(3-5-15)10-14(12-24)27-22(28)19-11-18(23)20(30-19)16-6-8-25-21-17(16)7-9-26-21/h2-9,11,14H,10,12,24H2,1H3,(H,25,26)(H,27,28)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278833
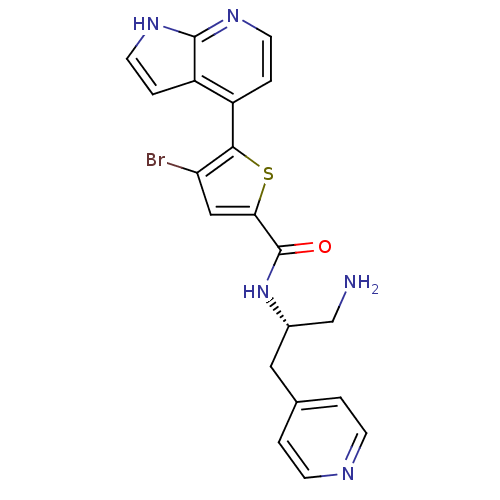
(CHEMBL498051 | N-((S)-1-amino-3-(pyridin-4-yl)prop...)Show SMILES NC[C@H](Cc1ccncc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C20H18BrN5OS/c21-16-10-17(20(27)26-13(11-22)9-12-1-5-23-6-2-12)28-18(16)14-3-7-24-19-15(14)4-8-25-19/h1-8,10,13H,9,11,22H2,(H,24,25)(H,26,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316185
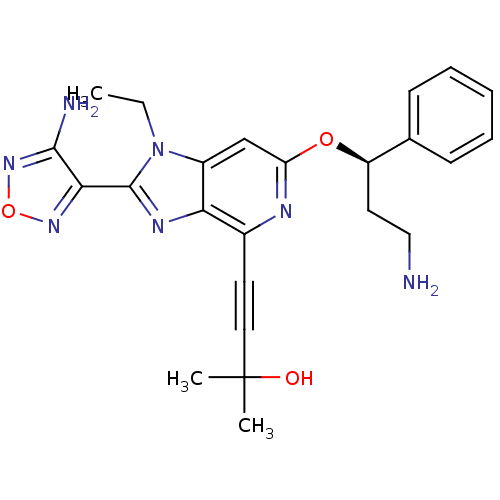
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278603
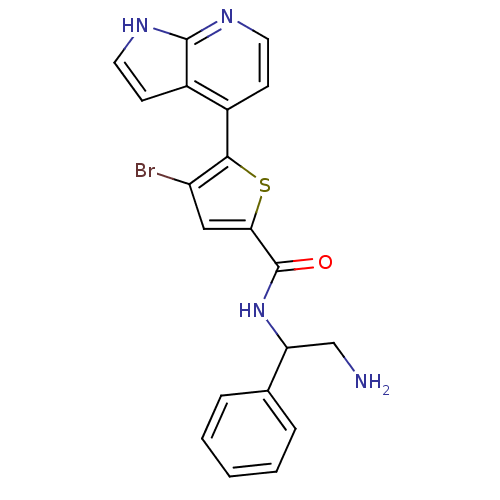
((+/-)-N-(2-amino-1-phenylethyl)-4-bromo-5-(1H-pyrr...)Show SMILES NCC(NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12)c1ccccc1 Show InChI InChI=1S/C20H17BrN4OS/c21-15-10-17(20(26)25-16(11-22)12-4-2-1-3-5-12)27-18(15)13-6-8-23-19-14(13)7-9-24-19/h1-10,16H,11,22H2,(H,23,24)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278603

((+/-)-N-(2-amino-1-phenylethyl)-4-bromo-5-(1H-pyrr...)Show SMILES NCC(NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12)c1ccccc1 Show InChI InChI=1S/C20H17BrN4OS/c21-15-10-17(20(26)25-16(11-22)12-4-2-1-3-5-12)27-18(15)13-6-8-23-19-14(13)7-9-24-19/h1-10,16H,11,22H2,(H,23,24)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15160

(1-[1-({4-[6-(2-methylpropyl)-5-oxo-3-phenyl-4,5-di...)Show SMILES CC(C)Cc1nc(-c2ccc(CN3CCC(CC3)n3c4ccccc4[nH]c3=O)cc2)c([nH]c1=O)-c1ccccc1 Show InChI InChI=1S/C33H35N5O2/c1-22(2)20-28-32(39)36-31(24-8-4-3-5-9-24)30(34-28)25-14-12-23(13-15-25)21-37-18-16-26(17-19-37)38-29-11-7-6-10-27(29)35-33(38)40/h3-15,22,26H,16-21H2,1-2H3,(H,35,40)(H,36,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of Akt2 |
Chem Biol 12: 621-37 (2005)
Article DOI: 10.1016/j.chembiol.2005.04.011
BindingDB Entry DOI: 10.7270/Q2R49RR6 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316197

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-(3-aminoprop...)Show SMILES CCn1c(nc2c(nc(OCCCN)cc12)C#CC(C)(C)O)-c1nonc1N Show InChI InChI=1S/C18H23N7O3/c1-4-25-12-10-13(27-9-5-8-19)21-11(6-7-18(2,3)26)14(12)22-17(25)15-16(20)24-28-23-15/h10,26H,4-5,8-9,19H2,1-3H3,(H2,20,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278769
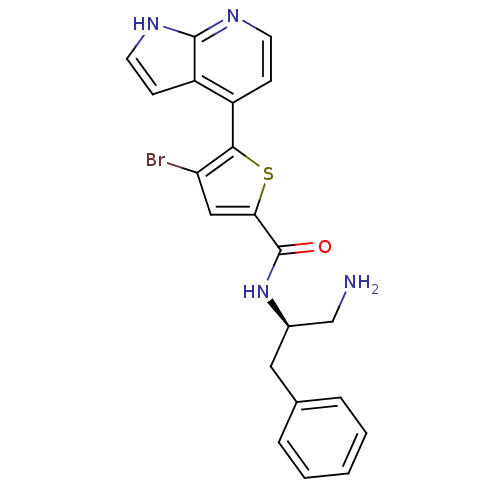
(CHEMBL513728 | N-((R)-1-amino-3-phenylpropan-2-yl)...)Show SMILES NC[C@@H](Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278769

(CHEMBL513728 | N-((R)-1-amino-3-phenylpropan-2-yl)...)Show SMILES NC[C@@H](Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha/RAC-beta/RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
RAC-alpha/RAC-beta/RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
RAC-alpha/RAC-beta/RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50224883
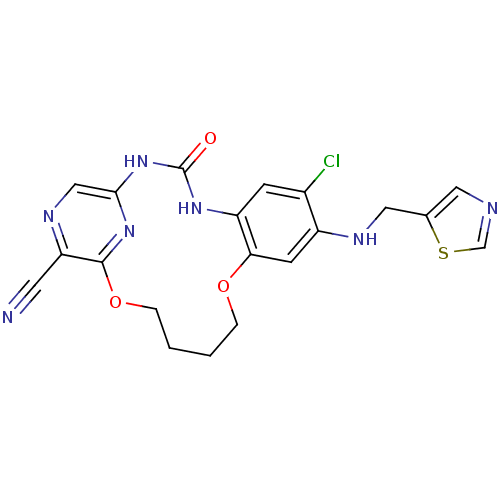
(7-chloro-3-oxo-8-[(thiazol-5-ylmethyl)-amino]-11,1...)Show SMILES Clc1cc2NC(=O)Nc3cnc(C#N)c(OCCCCOc2cc1NCc1cncs1)n3 Show InChI InChI=1S/C20H18ClN7O3S/c21-13-5-15-17(6-14(13)24-9-12-8-23-11-32-12)30-3-1-2-4-31-19-16(7-22)25-10-18(27-19)28-20(29)26-15/h5-6,8,10-11,24H,1-4,9H2,(H2,26,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 17: 6593-601 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.063
BindingDB Entry DOI: 10.7270/Q2X067WT |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50402020
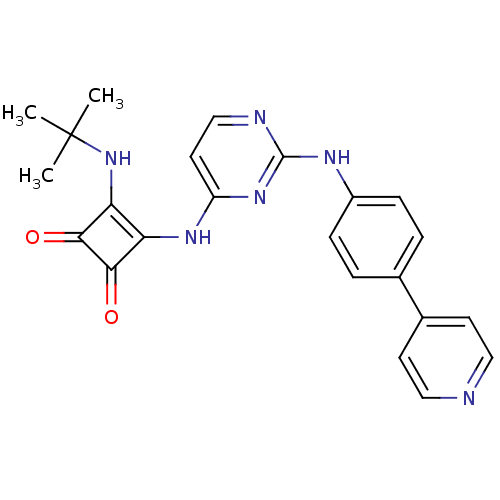
(CHEMBL2205426)Show SMILES CC(C)(C)Nc1c(Nc2ccnc(Nc3ccc(cc3)-c3ccncc3)n2)c(=O)c1=O Show InChI InChI=1S/C23H22N6O2/c1-23(2,3)29-19-18(20(30)21(19)31)27-17-10-13-25-22(28-17)26-16-6-4-14(5-7-16)15-8-11-24-12-9-15/h4-13,29H,1-3H3,(H2,25,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKT2 after 1 hr by scintillation counter analysis in presence of gamma-[33P]ATP |
Bioorg Med Chem Lett 22: 7615-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.009
BindingDB Entry DOI: 10.7270/Q2XK8GQ3 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50463484
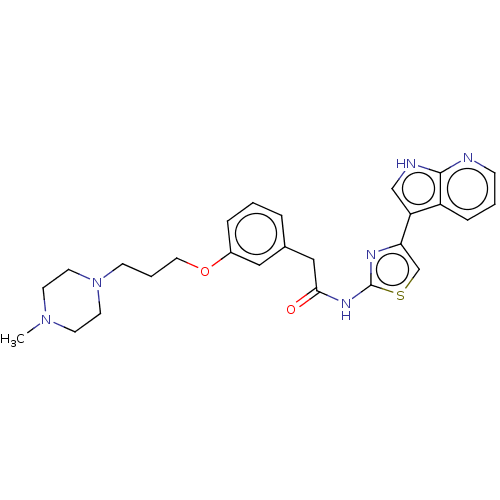
(CHEMBL4248525)Show SMILES CN1CCN(CCCOc2cccc(CC(=O)Nc3nc(cs3)-c3c[nH]c4ncccc34)c2)CC1 Show InChI InChI=1S/C26H30N6O2S/c1-31-10-12-32(13-11-31)9-4-14-34-20-6-2-5-19(15-20)16-24(33)30-26-29-23(18-35-26)22-17-28-25-21(22)7-3-8-27-25/h2-3,5-8,15,17-18H,4,9-14,16H2,1H3,(H,27,28)(H,29,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Akt2 (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50463479

(CHEMBL4249925)Show SMILES CS(=O)(=O)Nc1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C19H17N5O3S2/c1-29(26,27)24-13-5-2-4-12(8-13)9-17(25)23-19-22-16(11-28-19)15-10-21-18-14(15)6-3-7-20-18/h2-8,10-11,24H,9H2,1H3,(H,20,21)(H,22,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Akt2 (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50463483
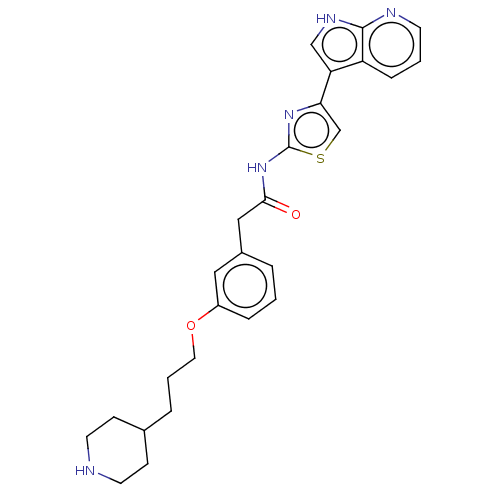
(CHEMBL4245242)Show SMILES O=C(Cc1cccc(OCCCC2CCNCC2)c1)Nc1nc(cs1)-c1c[nH]c2ncccc12 Show InChI InChI=1S/C26H29N5O2S/c32-24(31-26-30-23(17-34-26)22-16-29-25-21(22)7-2-10-28-25)15-19-4-1-6-20(14-19)33-13-3-5-18-8-11-27-12-9-18/h1-2,4,6-7,10,14,16-18,27H,3,5,8-9,11-13,15H2,(H,28,29)(H,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of Akt2 (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50528416
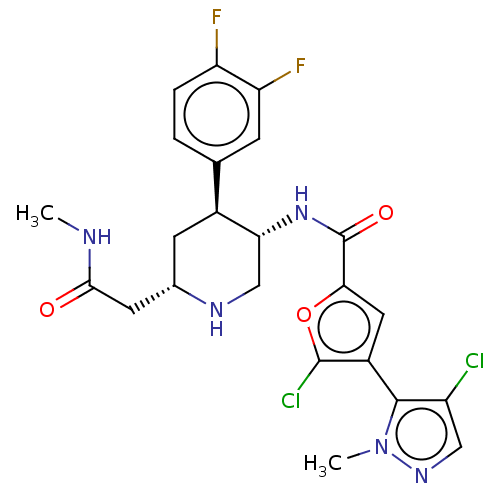
(CHEMBL4440965)Show SMILES CNC(=O)C[C@@H]1C[C@H]([C@@H](CN1)NC(=O)c1cc(c(Cl)o1)-c1c(Cl)cnn1C)c1ccc(F)c(F)c1 |r,wU:8.11,5.4,wD:7.29,(76.34,-11.8,;75,-12.56,;73.67,-11.79,;73.68,-10.25,;72.34,-12.55,;71.01,-11.78,;71.01,-10.23,;69.68,-9.47,;68.34,-10.24,;68.34,-11.77,;69.67,-12.55,;67.01,-9.46,;65.68,-10.23,;65.67,-11.77,;64.34,-9.46,;62.93,-10.08,;61.9,-8.93,;62.68,-7.6,;62.05,-6.19,;64.18,-7.92,;60.37,-9.09,;59.59,-10.42,;60.22,-11.83,;58.09,-10.1,;57.93,-8.57,;59.34,-7.94,;59.66,-6.44,;69.68,-7.94,;68.35,-7.17,;68.35,-5.62,;69.68,-4.85,;69.67,-3.31,;71.01,-5.62,;72.34,-4.84,;71.02,-7.17,)| Show InChI InChI=1S/C23H23Cl2F2N5O3/c1-28-20(33)7-12-6-13(11-3-4-16(26)17(27)5-11)18(10-29-12)31-23(34)19-8-14(22(25)35-19)21-15(24)9-30-32(21)2/h3-5,8-9,12-13,18,29H,6-7,10H2,1-2H3,(H,28,33)(H,31,34)/t12-,13-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal GST-tagged human AKT2 (120 to 481 residues) expressed in baculovirus infected Sf21 cells incubated for 1 hr in presence of A... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00815
BindingDB Entry DOI: 10.7270/Q2GF0Z92 |
More data for this
Ligand-Target Pair | |
RAC-alpha/RAC-beta/RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM431867
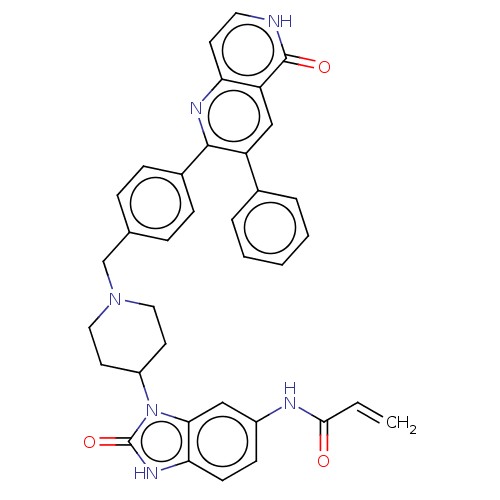
(US10550114, Compound 1a)Show SMILES C=CC(=O)Nc1ccc2[nH]c(=O)n(C3CCN(Cc4ccc(cc4)-c4nc5cc[nH]c(=O)c5cc4-c4ccccc4)CC3)c2c1 Show InChI InChI=1S/C36H32N6O3/c1-2-33(43)38-26-12-13-31-32(20-26)42(36(45)40-31)27-15-18-41(19-16-27)22-23-8-10-25(11-9-23)34-28(24-6-4-3-5-7-24)21-29-30(39-34)14-17-37-35(29)44/h2-14,17,20-21,27H,1,15-16,18-19,22H2,(H,37,44)(H,38,43)(H,40,45) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00073
BindingDB Entry DOI: 10.7270/Q2028WJ8 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM126609
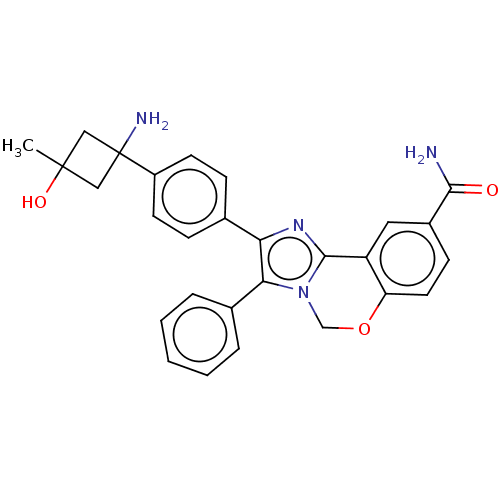
(US8772283, 53)Show SMILES CC1(O)CC(N)(C1)c1ccc(cc1)-c1nc2-c3cc(ccc3OCn2c1-c1ccccc1)C(N)=O Show InChI InChI=1S/C28H26N4O3/c1-27(34)14-28(30,15-27)20-10-7-17(8-11-20)23-24(18-5-3-2-4-6-18)32-16-35-22-12-9-19(25(29)33)13-21(22)26(32)31-23/h2-13,34H,14-16,30H2,1H3,(H2,29,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... |
US Patent US8772283 (2014)
BindingDB Entry DOI: 10.7270/Q21J98F0 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM126581
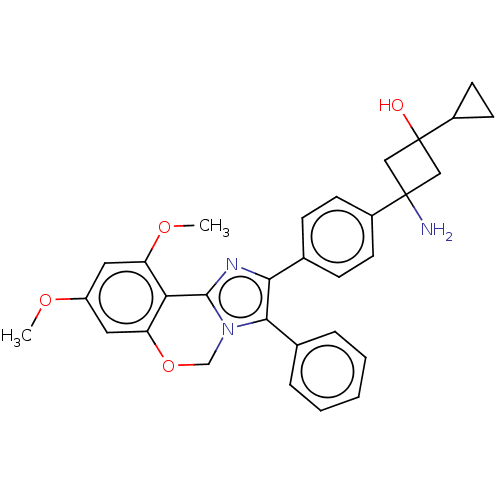
(US8772283, 25)Show SMILES COc1cc(OC)c2-c3nc(c(-c4ccccc4)n3COc2c1)-c1ccc(cc1)C1(N)CC(O)(C1)C1CC1 |(-8.4,-.89,;-7.63,.45,;-6.09,.45,;-5.32,1.78,;-3.78,1.78,;-2.69,2.87,;-3.09,4.36,;-3.01,.45,;-1.47,.45,;-.5,1.64,;.93,1.09,;.85,-.45,;2.06,-1.41,;1.66,-2.89,;2.75,-3.98,;4.23,-3.58,;4.63,-2.1,;3.54,-1.01,;-.7,-.89,;-1.47,-2.22,;-3.01,-2.22,;-3.78,-.89,;-5.32,-.89,;2.23,1.93,;2.23,3.47,;3.56,4.24,;4.89,3.47,;4.89,1.93,;3.56,1.16,;6.23,4.24,;7.56,3.47,;5.14,5.33,;6.31,6.43,;6.36,7.97,;7.32,5.33,;4.9,7.07,;3.57,6.3,;4.13,8.4,)| Show InChI InChI=1S/C31H31N3O4/c1-36-23-14-24(37-2)26-25(15-23)38-18-34-28(20-6-4-3-5-7-20)27(33-29(26)34)19-8-10-21(11-9-19)30(32)16-31(35,17-30)22-12-13-22/h3-11,14-15,22,35H,12-13,16-18,32H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... |
US Patent US8772283 (2014)
BindingDB Entry DOI: 10.7270/Q21J98F0 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
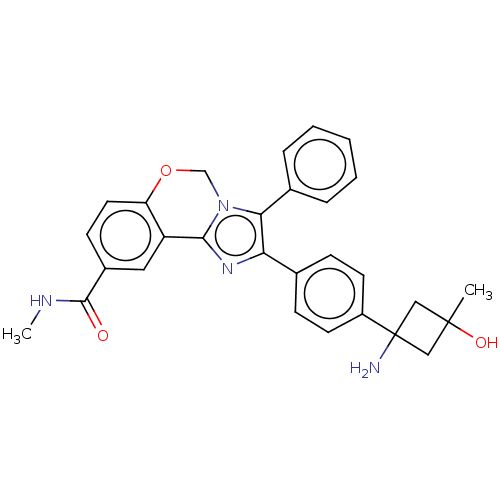
(Homo sapiens (Human)) | BDBM126608
(US8772283, 52)Show SMILES CNC(=O)c1ccc2OCn3c(nc(c3-c3ccccc3)-c3ccc(cc3)C3(N)CC(C)(O)C3)-c2c1 Show InChI InChI=1S/C29H28N4O3/c1-28(35)15-29(30,16-28)21-11-8-18(9-12-21)24-25(19-6-4-3-5-7-19)33-17-36-23-13-10-20(27(34)31-2)14-22(23)26(33)32-24/h3-14,35H,15-17,30H2,1-2H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... |
US Patent US8772283 (2014)
BindingDB Entry DOI: 10.7270/Q21J98F0 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
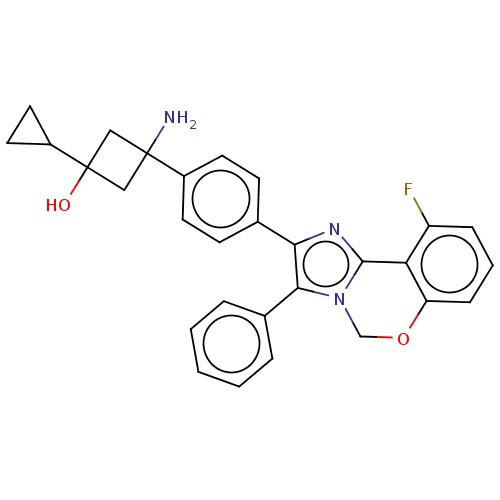
(Homo sapiens (Human)) | BDBM126557
(US8772283, 1)Show SMILES NC1(CC(O)(C1)C1CC1)c1ccc(cc1)-c1nc2-c3c(F)cccc3OCn2c1-c1ccccc1 |(7.56,3.47,;6.23,4.24,;5.14,5.33,;6.31,6.43,;6.36,7.97,;7.32,5.33,;4.9,7.07,;3.57,6.3,;4.13,8.4,;4.89,3.47,;3.56,4.24,;2.23,3.47,;2.23,1.93,;3.56,1.16,;4.89,1.93,;.93,1.09,;-.5,1.64,;-1.47,.45,;-3.01,.45,;-3.78,1.78,;-2.69,2.87,;-5.32,1.78,;-6.09,.45,;-5.32,-.89,;-3.78,-.89,;-3.01,-2.22,;-1.47,-2.22,;-.7,-.89,;.85,-.45,;2.06,-1.41,;1.66,-2.89,;2.75,-3.98,;4.23,-3.58,;4.63,-2.1,;3.54,-1.01,)| Show InChI InChI=1S/C29H26FN3O2/c30-22-7-4-8-23-24(22)27-32-25(26(33(27)17-35-23)19-5-2-1-3-6-19)18-9-11-20(12-10-18)28(31)15-29(34,16-28)21-13-14-21/h1-12,21,34H,13-17,31H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... |
US Patent US8772283 (2014)
BindingDB Entry DOI: 10.7270/Q21J98F0 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM126578

(US8772283, 22)Show SMILES NC1(CC(O)(C1)C1CC1)c1ccc(cc1)-c1nc2-c3c(Cl)cccc3OCn2c1-c1ccccc1 |(7.56,3.47,;6.23,4.24,;5.14,5.33,;6.31,6.43,;6.36,7.97,;7.32,5.33,;4.9,7.07,;3.57,6.3,;4.13,8.4,;4.89,3.47,;3.56,4.24,;2.23,3.47,;2.23,1.93,;3.56,1.16,;4.89,1.93,;.93,1.09,;-.5,1.64,;-1.47,.45,;-3.01,.45,;-3.78,1.78,;-2.69,2.87,;-5.32,1.78,;-6.09,.45,;-5.32,-.89,;-3.78,-.89,;-3.01,-2.22,;-1.47,-2.22,;-.7,-.89,;.85,-.45,;2.06,-1.41,;1.66,-2.89,;2.75,-3.98,;4.23,-3.58,;4.63,-2.1,;3.54,-1.01,)| Show InChI InChI=1S/C29H26ClN3O2/c30-22-7-4-8-23-24(22)27-32-25(26(33(27)17-35-23)19-5-2-1-3-6-19)18-9-11-20(12-10-18)28(31)15-29(34,16-28)21-13-14-21/h1-12,21,34H,13-17,31H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... |
US Patent US8772283 (2014)
BindingDB Entry DOI: 10.7270/Q21J98F0 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM126611

(US8772283, 55)Show SMILES CCNC(=O)c1ccc2OCn3c(nc(c3-c3ccccc3)-c3ccc(cc3)C3(N)CC(C)(O)C3)-c2c1 Show InChI InChI=1S/C30H30N4O3/c1-3-32-28(35)21-11-14-24-23(15-21)27-33-25(26(34(27)18-37-24)20-7-5-4-6-8-20)19-9-12-22(13-10-19)30(31)16-29(2,36)17-30/h4-15,36H,3,16-18,31H2,1-2H3,(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... |
US Patent US8772283 (2014)
BindingDB Entry DOI: 10.7270/Q21J98F0 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
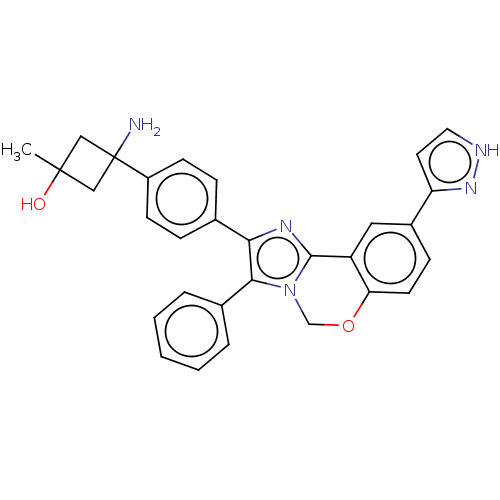
(Homo sapiens (Human)) | BDBM126604
(US8772283, 48)Show SMILES CC1(O)CC(N)(C1)c1ccc(cc1)-c1nc2-c3cc(ccc3OCn2c1-c1ccccc1)-c1ccn[nH]1 Show InChI InChI=1S/C30H27N5O2/c1-29(36)16-30(31,17-29)22-10-7-19(8-11-22)26-27(20-5-3-2-4-6-20)35-18-37-25-12-9-21(24-13-14-32-34-24)15-23(25)28(35)33-26/h2-15,36H,16-18,31H2,1H3,(H,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... |
US Patent US8772283 (2014)
BindingDB Entry DOI: 10.7270/Q21J98F0 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM126572
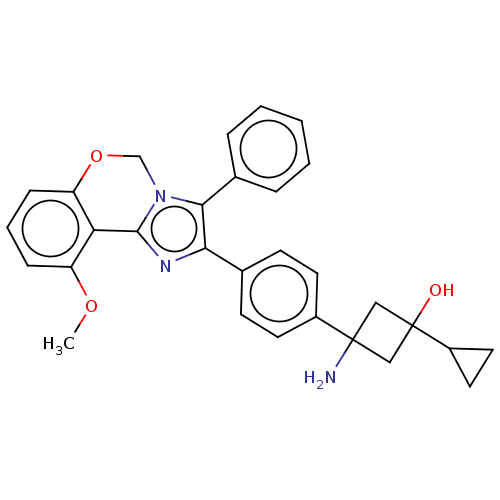
(US8772283, 16)Show SMILES COc1cccc2OCn3c(nc(c3-c3ccccc3)-c3ccc(cc3)C3(N)CC(O)(C3)C3CC3)-c12 |(-3.09,4.36,;-2.69,2.87,;-3.78,1.78,;-5.32,1.78,;-6.09,.45,;-5.32,-.89,;-3.78,-.89,;-3.01,-2.22,;-1.47,-2.22,;-.7,-.89,;-1.47,.45,;-.5,1.64,;.93,1.09,;.85,-.45,;2.06,-1.41,;1.66,-2.89,;2.75,-3.98,;4.23,-3.58,;4.63,-2.1,;3.54,-1.01,;2.23,1.93,;2.23,3.47,;3.56,4.24,;4.89,3.47,;4.89,1.93,;3.56,1.16,;6.23,4.24,;7.56,3.47,;5.14,5.33,;6.31,6.43,;6.36,7.97,;7.32,5.33,;4.9,7.07,;3.57,6.3,;4.13,8.4,;-3.01,.45,)| Show InChI InChI=1S/C30H29N3O3/c1-35-23-8-5-9-24-25(23)28-32-26(27(33(28)18-36-24)20-6-3-2-4-7-20)19-10-12-21(13-11-19)29(31)16-30(34,17-29)22-14-15-22/h2-13,22,34H,14-18,31H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... |
US Patent US8772283 (2014)
BindingDB Entry DOI: 10.7270/Q21J98F0 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM126618

(US8772283, 62)Show SMILES CC1(O)CC(N)(C1)c1ccc(cc1)-c1nc2-c3ccc(cc3OCn2c1-c1ccccc1)C(N)=O Show InChI InChI=1S/C28H26N4O3/c1-27(34)14-28(30,15-27)20-10-7-17(8-11-20)23-24(18-5-3-2-4-6-18)32-16-35-22-13-19(25(29)33)9-12-21(22)26(32)31-23/h2-13,34H,14-16,30H2,1H3,(H2,29,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... |
US Patent US8772283 (2014)
BindingDB Entry DOI: 10.7270/Q21J98F0 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM126617

(US8772283, 61)Show SMILES CCONC(=O)c1ccc2OCn3c(nc(c3-c3ccccc3)-c3ccc(cc3)C3(N)CC(C)(O)C3)-c2c1 Show InChI InChI=1S/C30H30N4O4/c1-3-38-33-28(35)21-11-14-24-23(15-21)27-32-25(26(34(27)18-37-24)20-7-5-4-6-8-20)19-9-12-22(13-10-19)30(31)16-29(2,36)17-30/h4-15,36H,3,16-18,31H2,1-2H3,(H,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... |
US Patent US8772283 (2014)
BindingDB Entry DOI: 10.7270/Q21J98F0 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM126605
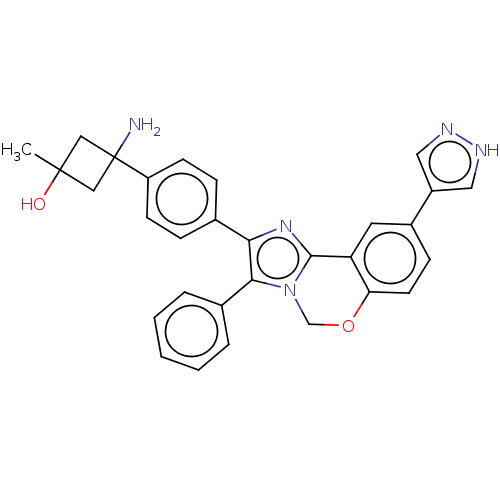
(US8772283, 49)Show SMILES CC1(O)CC(N)(C1)c1ccc(cc1)-c1nc2-c3cc(ccc3OCn2c1-c1ccccc1)-c1cn[nH]c1 Show InChI InChI=1S/C30H27N5O2/c1-29(36)16-30(31,17-29)23-10-7-19(8-11-23)26-27(20-5-3-2-4-6-20)35-18-37-25-12-9-21(22-14-32-33-15-22)13-24(25)28(35)34-26/h2-15,36H,16-18,31H2,1H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... |
US Patent US8772283 (2014)
BindingDB Entry DOI: 10.7270/Q21J98F0 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM126591

(US8772283, 35)Show SMILES NC1(CC(O)(C1)C1CC1)c1ccc(cc1)-c1nc2-c3ccncc3OCn2c1-c1ccccc1 |(7.56,3.47,;6.23,4.24,;5.14,5.33,;6.31,6.43,;6.36,7.97,;7.32,5.33,;4.9,7.07,;3.57,6.3,;4.13,8.4,;4.89,3.47,;3.56,4.24,;2.23,3.47,;2.23,1.93,;3.56,1.16,;4.89,1.93,;.93,1.09,;-.5,1.64,;-1.47,.45,;-3.01,.45,;-3.78,1.78,;-5.32,1.78,;-6.09,.45,;-5.32,-.89,;-3.78,-.89,;-3.01,-2.22,;-1.47,-2.22,;-.7,-.89,;.85,-.45,;2.06,-1.41,;1.66,-2.89,;2.75,-3.98,;4.23,-3.58,;4.63,-2.1,;3.54,-1.01,)| Show InChI InChI=1S/C28H26N4O2/c29-27(15-28(33,16-27)21-10-11-21)20-8-6-18(7-9-20)24-25(19-4-2-1-3-5-19)32-17-34-23-14-30-13-12-22(23)26(32)31-24/h1-9,12-14,21,33H,10-11,15-17,29H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... |
US Patent US8772283 (2014)
BindingDB Entry DOI: 10.7270/Q21J98F0 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM126612
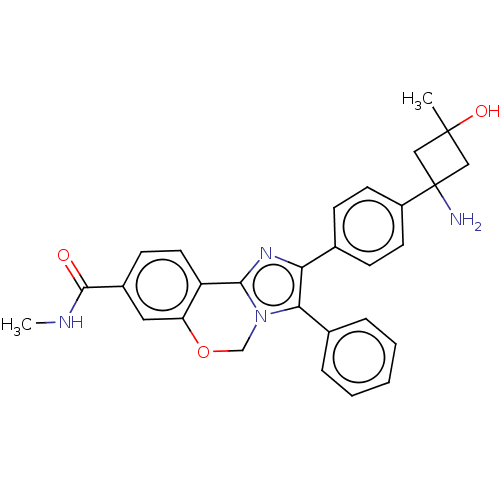
(US8772283, 56)Show SMILES CNC(=O)c1ccc2-c3nc(c(-c4ccccc4)n3COc2c1)-c1ccc(cc1)C1(N)CC(C)(O)C1 Show InChI InChI=1S/C29H28N4O3/c1-28(35)15-29(30,16-28)21-11-8-18(9-12-21)24-25(19-6-4-3-5-7-19)33-17-36-23-14-20(27(34)31-2)10-13-22(23)26(33)32-24/h3-14,35H,15-17,30H2,1-2H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... |
US Patent US8772283 (2014)
BindingDB Entry DOI: 10.7270/Q21J98F0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data