 Found 424 hits for UniProtKB: P42684
Found 424 hits for UniProtKB: P42684 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50020310
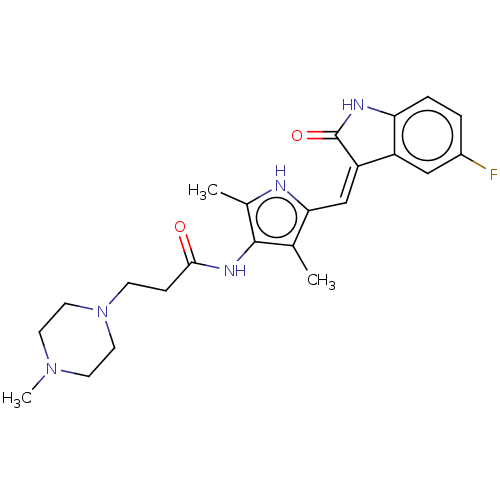
(CHEMBL3288854)Show SMILES CN1CCN(CCC(=O)Nc2c(C)[nH]c(\C=C3/C(=O)Nc4ccc(F)cc34)c2C)CC1 Show InChI InChI=1S/C23H28FN5O2/c1-14-20(13-18-17-12-16(24)4-5-19(17)26-23(18)31)25-15(2)22(14)27-21(30)6-7-29-10-8-28(3)9-11-29/h4-5,12-13,25H,6-11H2,1-3H3,(H,26,31)(H,27,30)/b18-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qilu Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to ARG (unknown origin) |
Eur J Med Chem 82: 139-51 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.051
BindingDB Entry DOI: 10.7270/Q2F1918D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50331612
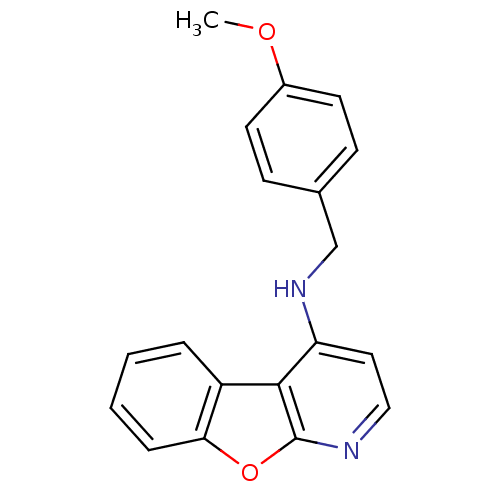
(CHEMBL1290072 | N-(4-methoxybenzyl)benzofuro[2,3-b...)Show InChI InChI=1S/C19H16N2O2/c1-22-14-8-6-13(7-9-14)12-21-16-10-11-20-19-18(16)15-4-2-3-5-17(15)23-19/h2-11H,12H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin Luther University Halle-Wittenberg
Curated by ChEMBL
| Assay Description
Inhibition of human ABL2 |
Bioorg Med Chem Lett 20: 6915-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.004
BindingDB Entry DOI: 10.7270/Q2GF0TQS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Molecular Medicine of the Austrian Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of ABL2 |
Leukemia 23: 477-85 (2009)
Article DOI: 10.1038/leu.2008.334
BindingDB Entry DOI: 10.7270/Q22Z15R6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM6096
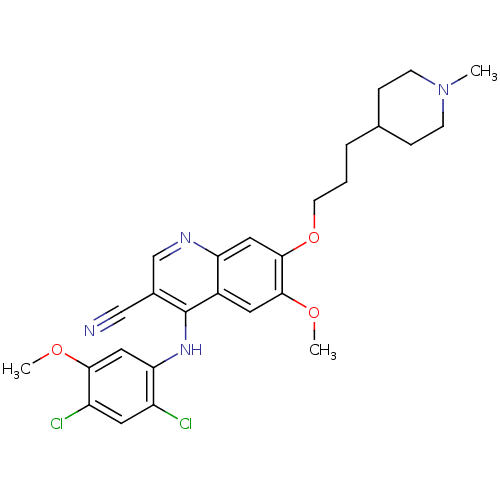
(3-quinolinecarbonitrile 24 | 4-[(2,4-Dichloro-5-me...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCC4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C27H30Cl2N4O3/c1-33-8-6-17(7-9-33)5-4-10-36-26-13-22-19(11-25(26)35-3)27(18(15-30)16-31-22)32-23-14-24(34-2)21(29)12-20(23)28/h11-14,16-17H,4-10H2,1-3H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Abl kinase |
J Med Chem 47: 1599-601 (2004)
Article DOI: 10.1021/jm0499458
BindingDB Entry DOI: 10.7270/Q2BP05HD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427749
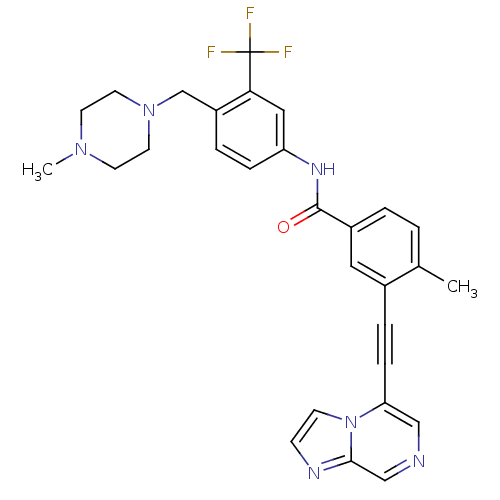
(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Abl kinase |
J Med Chem 47: 1599-601 (2004)
Article DOI: 10.1021/jm0499458
BindingDB Entry DOI: 10.7270/Q2BP05HD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427749

(CHEMBL2324924)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cncc4nccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-33-18-27-34-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM6095

(3-quinolinecarbonitrile 23 | 4-[(2,4-Dichloro-5-me...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCC4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H28Cl2N4O3/c1-32-7-4-16(5-8-32)6-9-35-25-12-21-18(10-24(25)34-3)26(17(14-29)15-30-21)31-22-13-23(33-2)20(28)11-19(22)27/h10-13,15-16H,4-9H2,1-3H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Abl kinase |
J Med Chem 47: 1599-601 (2004)
Article DOI: 10.1021/jm0499458
BindingDB Entry DOI: 10.7270/Q2BP05HD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM6094

(3-quinolinecarbonitrile 18 | 4-[(2,4-Dichloro-5-me...)Show SMILES COc1cc(Nc2c(cnc3cc(OCC4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C25H26Cl2N4O3/c1-31-6-4-15(5-7-31)14-34-24-10-20-17(8-23(24)33-3)25(16(12-28)13-29-20)30-21-11-22(32-2)19(27)9-18(21)26/h8-11,13,15H,4-7,14H2,1-3H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Abl kinase |
J Med Chem 47: 1599-601 (2004)
Article DOI: 10.1021/jm0499458
BindingDB Entry DOI: 10.7270/Q2BP05HD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427748
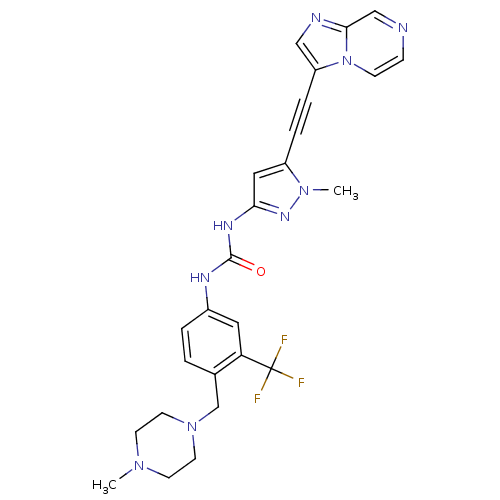
(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM6102
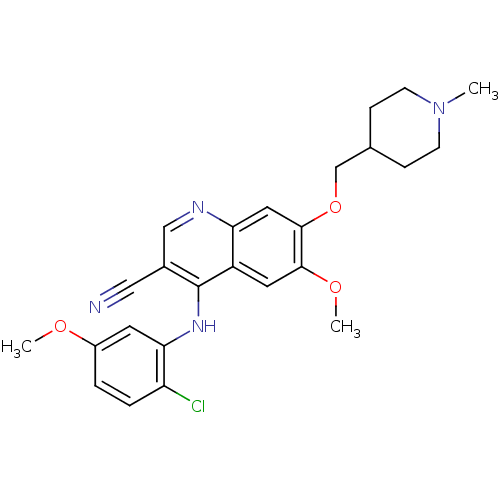
(4-[(2-Chloro-5-methoxyphenyl)amino]-6-methoxy-7-[(...)Show SMILES COc1ccc(Cl)c(Nc2c(cnc3cc(OCC4CCN(C)CC4)c(OC)cc23)C#N)c1 Show InChI InChI=1S/C25H27ClN4O3/c1-30-8-6-16(7-9-30)15-33-24-12-21-19(11-23(24)32-3)25(17(13-27)14-28-21)29-22-10-18(31-2)4-5-20(22)26/h4-5,10-12,14,16H,6-9,15H2,1-3H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Abl kinase |
J Med Chem 47: 1599-601 (2004)
Article DOI: 10.1021/jm0499458
BindingDB Entry DOI: 10.7270/Q2BP05HD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG (38 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radio... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
| Assay Description
The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... |
Bioorg Med Chem Lett 17: 3562-9 (2007)
BindingDB Entry DOI: 10.7270/Q21C2065 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM6099

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCC4CCCN(C)C4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C25H26Cl2N4O3/c1-31-6-4-5-15(13-31)14-34-24-9-20-17(7-23(24)33-3)25(16(11-28)12-29-20)30-21-10-22(32-2)19(27)8-18(21)26/h7-10,12,15H,4-6,13-14H2,1-3H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Abl kinase |
J Med Chem 47: 1599-601 (2004)
Article DOI: 10.1021/jm0499458
BindingDB Entry DOI: 10.7270/Q2BP05HD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM50097942

(3-(4-Amino-7-cyclobutyl-7H-pyrrolo[2,3-d]pyrimidin...)Show InChI InChI=1S/C16H16N4O/c17-15-14-13(10-3-1-6-12(21)7-10)8-20(11-4-2-5-11)16(14)19-9-18-15/h1,3,6-9,11,21H,2,4-5H2,(H2,17,18,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research
Curated by ChEMBL
| Assay Description
Inhibition of v-Abl tyrosine kinase activity |
Bioorg Med Chem Lett 11: 849-52 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RMF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427747
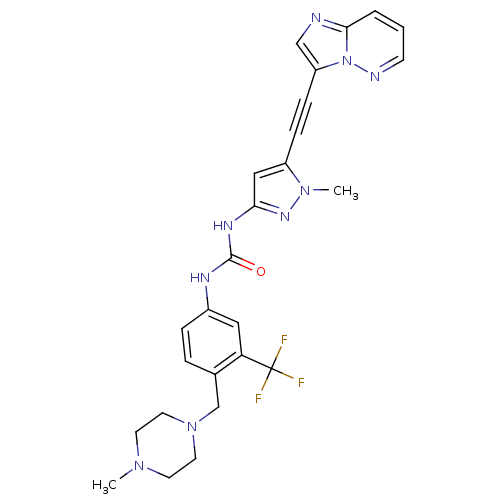
(CHEMBL2324926)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cccnn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-10-12-37(13-11-35)17-18-5-6-19(14-22(18)26(27,28)29)32-25(39)33-23-15-20(36(2)34-23)7-8-21-16-30-24-4-3-9-31-38(21)24/h3-6,9,14-16H,10-13,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427742
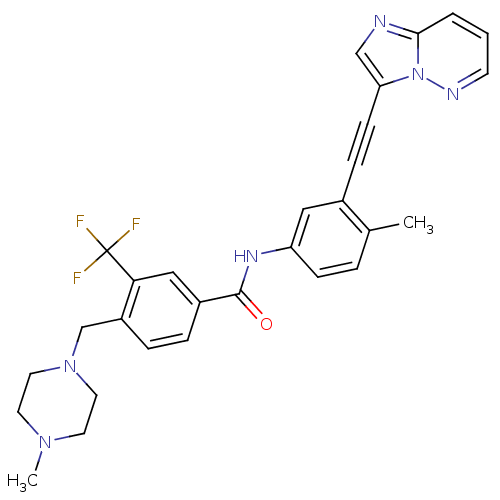
(CHEMBL2324930)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(c2)C#Cc2cnc3cccnn23)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-9-24(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)35-28(39)22-6-7-23(26(17-22)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM6105
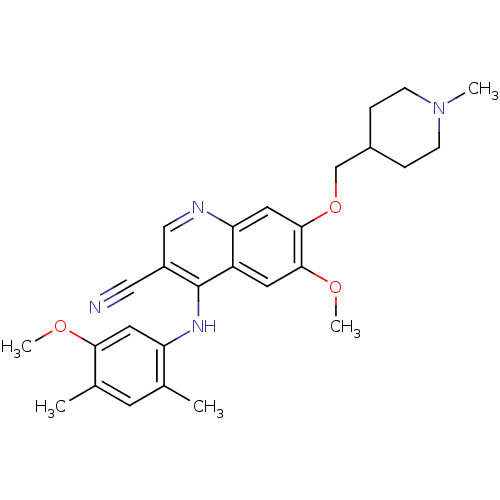
(4-phenylamino-3-quinolinecarbonitrile 23 | 6-Metho...)Show SMILES COc1cc(Nc2c(cnc3cc(OCC4CCN(C)CC4)c(OC)cc23)C#N)c(C)cc1C Show InChI InChI=1S/C27H32N4O3/c1-17-10-18(2)24(32-4)12-22(17)30-27-20(14-28)15-29-23-13-26(25(33-5)11-21(23)27)34-16-19-6-8-31(3)9-7-19/h10-13,15,19H,6-9,16H2,1-5H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Abl kinase |
J Med Chem 47: 1599-601 (2004)
Article DOI: 10.1021/jm0499458
BindingDB Entry DOI: 10.7270/Q2BP05HD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
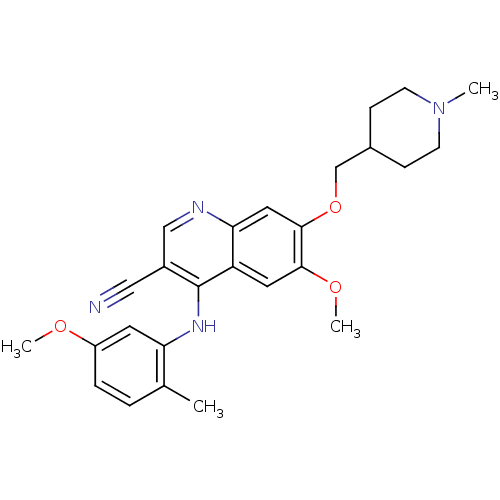
(Homo sapiens (Human)) | BDBM6103
(4-phenylamino-3-quinolinecarbonitrile 21 | 6-Metho...)Show SMILES COc1ccc(C)c(Nc2c(cnc3cc(OCC4CCN(C)CC4)c(OC)cc23)C#N)c1 Show InChI InChI=1S/C26H30N4O3/c1-17-5-6-20(31-3)11-22(17)29-26-19(14-27)15-28-23-13-25(24(32-4)12-21(23)26)33-16-18-7-9-30(2)10-8-18/h5-6,11-13,15,18H,7-10,16H2,1-4H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Abl kinase |
J Med Chem 47: 1599-601 (2004)
Article DOI: 10.1021/jm0499458
BindingDB Entry DOI: 10.7270/Q2BP05HD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
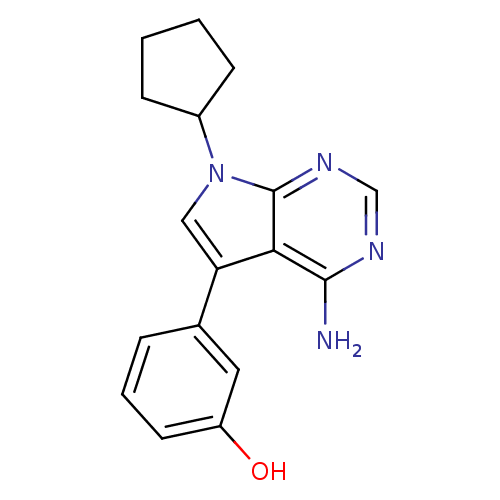
(Homo sapiens (Human)) | BDBM50097956
(3-(4-Amino-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrimidi...)Show InChI InChI=1S/C17H18N4O/c18-16-15-14(11-4-3-7-13(22)8-11)9-21(12-5-1-2-6-12)17(15)20-10-19-16/h3-4,7-10,12,22H,1-2,5-6H2,(H2,18,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research
Curated by ChEMBL
| Assay Description
Inhibition of v-Abl tyrosine kinase activity |
Bioorg Med Chem Lett 11: 849-52 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RMF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427748

(CHEMBL2324925)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cnccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-31-24-16-30-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ABL2 using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427750
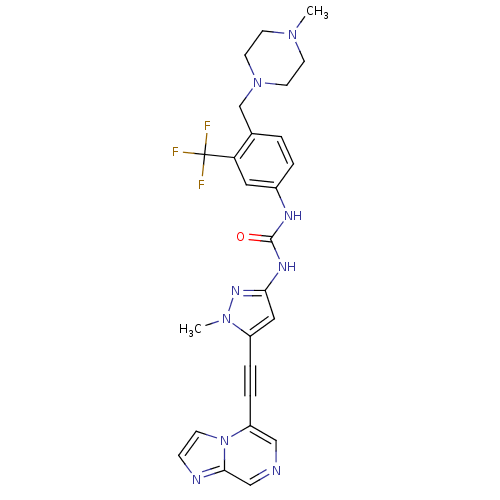
(CHEMBL2324923)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cncc5nccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-30-16-24-31-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427745
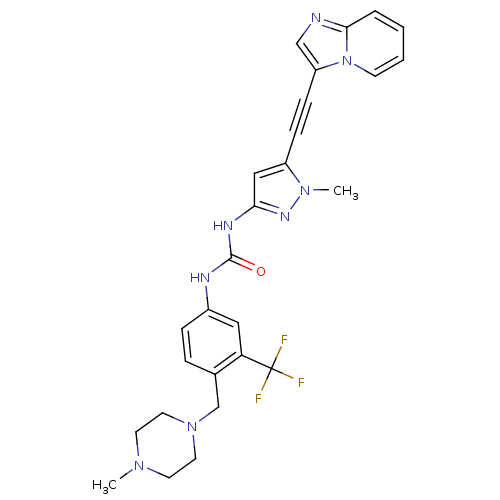
(CHEMBL2324927)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5ccccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C27H27F3N8O/c1-35-11-13-37(14-12-35)18-19-6-7-20(15-23(19)27(28,29)30)32-26(39)33-24-16-21(36(2)34-24)8-9-22-17-31-25-5-3-4-10-38(22)25/h3-7,10,15-17H,11-14,18H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM6101
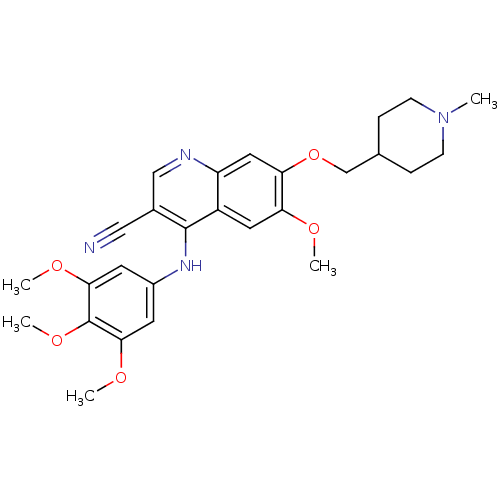
(3-quinolinecarbonitrile 20 | 4-phenylamino-3-quino...)Show SMILES COc1cc2c(Nc3cc(OC)c(OC)c(OC)c3)c(cnc2cc1OCC1CCN(C)CC1)C#N Show InChI InChI=1S/C27H32N4O5/c1-31-8-6-17(7-9-31)16-36-23-13-21-20(12-22(23)32-2)26(18(14-28)15-29-21)30-19-10-24(33-3)27(35-5)25(11-19)34-4/h10-13,15,17H,6-9,16H2,1-5H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Abl kinase |
J Med Chem 47: 1599-601 (2004)
Article DOI: 10.1021/jm0499458
BindingDB Entry DOI: 10.7270/Q2BP05HD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427744
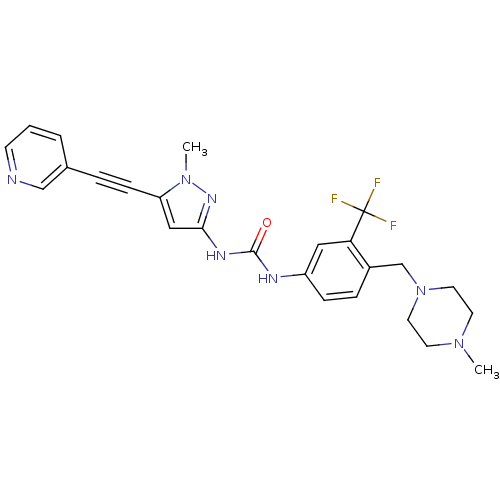
(CHEMBL2324928)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cccnc4)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C25H26F3N7O/c1-33-10-12-35(13-11-33)17-19-6-7-20(14-22(19)25(26,27)28)30-24(36)31-23-15-21(34(2)32-23)8-5-18-4-3-9-29-16-18/h3-4,6-7,9,14-16H,10-13,17H2,1-2H3,(H2,30,31,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427750

(CHEMBL2324923)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cncc5nccn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-9-11-37(12-10-35)17-18-3-4-19(13-22(18)26(27,28)29)32-25(39)33-23-14-20(36(2)34-23)5-6-21-15-30-16-24-31-7-8-38(21)24/h3-4,7-8,13-16H,9-12,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ABL2 using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM6100
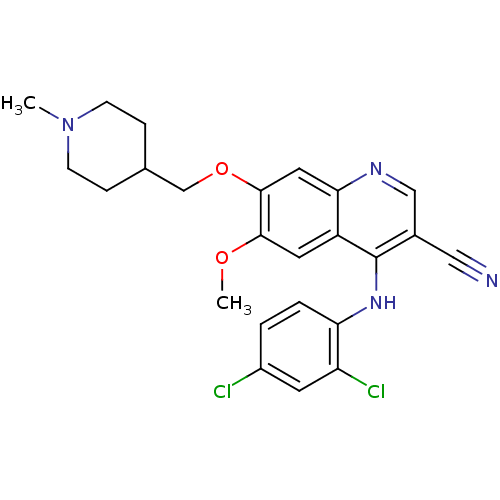
(3-quinolinecarbonitrile 19 | 4-[(2,4-Dichloropheny...)Show SMILES COc1cc2c(Nc3ccc(Cl)cc3Cl)c(cnc2cc1OCC1CCN(C)CC1)C#N Show InChI InChI=1S/C24H24Cl2N4O2/c1-30-7-5-15(6-8-30)14-32-23-11-21-18(10-22(23)31-2)24(16(12-27)13-28-21)29-20-4-3-17(25)9-19(20)26/h3-4,9-11,13,15H,5-8,14H2,1-2H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Abl kinase |
J Med Chem 47: 1599-601 (2004)
Article DOI: 10.1021/jm0499458
BindingDB Entry DOI: 10.7270/Q2BP05HD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427741
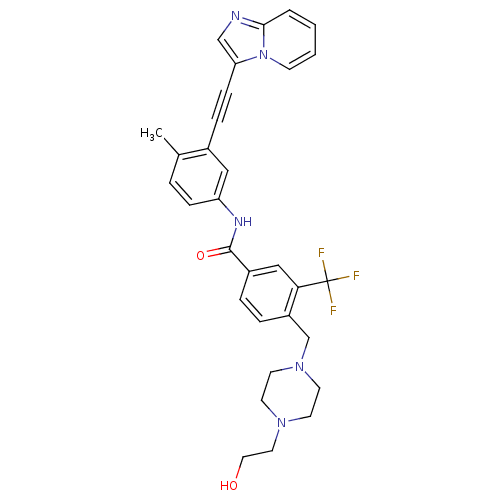
(CHEMBL2324931)Show SMILES Cc1ccc(NC(=O)c2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)cc1C#Cc1cnc2ccccn12 Show InChI InChI=1S/C31H30F3N5O2/c1-22-5-9-26(18-23(22)8-10-27-20-35-29-4-2-3-11-39(27)29)36-30(41)24-6-7-25(28(19-24)31(32,33)34)21-38-14-12-37(13-15-38)16-17-40/h2-7,9,11,18-20,40H,12-17,21H2,1H3,(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM50088900

(5,7-Diphenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine ...)Show InChI InChI=1S/C18H14N4/c19-17-16-15(13-7-3-1-4-8-13)11-22(18(16)21-12-20-17)14-9-5-2-6-10-14/h1-12H,(H2,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of PDGF-receptor kinase |
Bioorg Med Chem Lett 10: 945-9 (2000)
BindingDB Entry DOI: 10.7270/Q2R78DG4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM612783
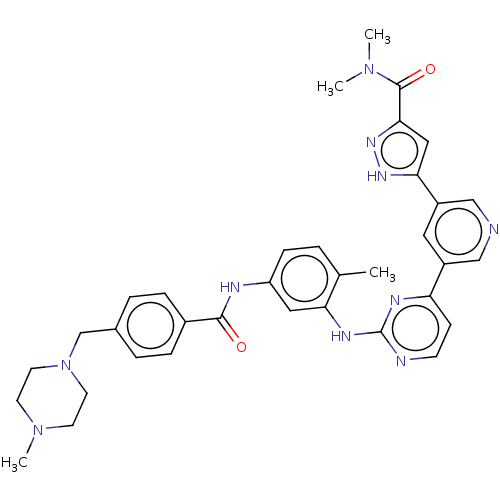
(US11725005, Compound 117)Show SMILES CN(C)C(=O)c1cc([nH]n1)-c1cncc(c1)-c1ccnc(Nc2cc(NC(=O)c3ccc(CN4CCN(C)CC4)cc3)ccc2C)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R215H9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM481113
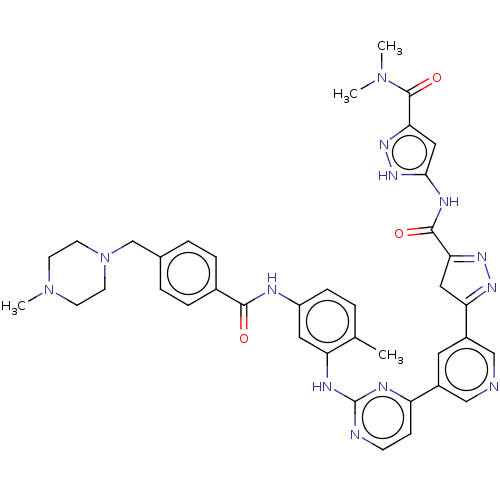
(US10906896, Cpd 117)Show SMILES CN(C)C(=O)c1cc(NC(=O)C2=NN=C(C2)c2cncc(c2)-c2ccnc(Nc3cc(NC(=O)c4ccc(CN5CCN(C)CC5)cc4)ccc3C)n2)[nH]n1 |c:13,t:11| Show InChI InChI=1S/C39H41N13O3/c1-24-5-10-29(42-36(53)26-8-6-25(7-9-26)23-52-15-13-51(4)14-16-52)18-31(24)44-39-41-12-11-30(43-39)27-17-28(22-40-21-27)32-19-33(47-46-32)37(54)45-35-20-34(48-49-35)38(55)50(2)3/h5-12,17-18,20-22H,13-16,19,23H2,1-4H3,(H,42,53)(H,41,43,44)(H2,45,48,49,54) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Inhibikase Therapeutics, Inc.
US Patent
| Assay Description
Kinase base buffer (50 mM HEPES, pH 7.5 0.0015% Brij-35; 10 mM MgCl2 2 mM DTT) and Stop buffer, (100 mM HEPES, pH 7.5 0.015% Brij-35; 0.2% Coating Re... |
US Patent US10906896 (2021)
BindingDB Entry DOI: 10.7270/Q2G44TCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427743
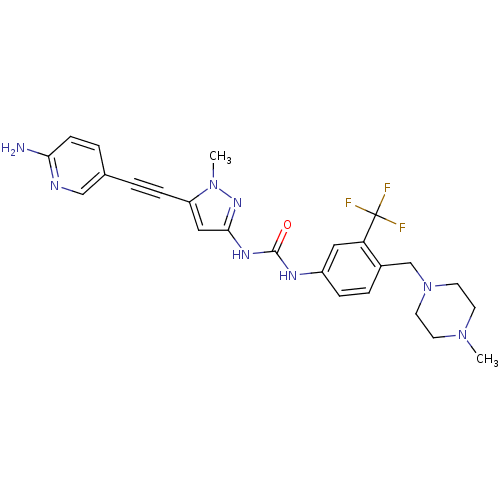
(CHEMBL2324929)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4ccc(N)nc4)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C25H27F3N8O/c1-34-9-11-36(12-10-34)16-18-5-6-19(13-21(18)25(26,27)28)31-24(37)32-23-14-20(35(2)33-23)7-3-17-4-8-22(29)30-15-17/h4-6,8,13-15H,9-12,16H2,1-2H3,(H2,29,30)(H2,31,32,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM6104
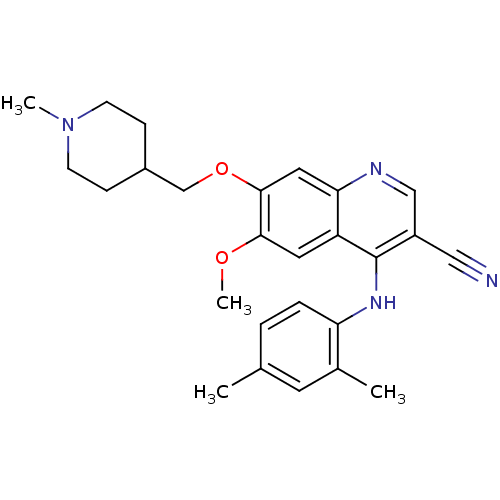
(4-[(2,4-Dimethylphenyl)amino]-6-methoxy-7-[(1-meth...)Show SMILES COc1cc2c(Nc3ccc(C)cc3C)c(cnc2cc1OCC1CCN(C)CC1)C#N Show InChI InChI=1S/C26H30N4O2/c1-17-5-6-22(18(2)11-17)29-26-20(14-27)15-28-23-13-25(24(31-4)12-21(23)26)32-16-19-7-9-30(3)10-8-19/h5-6,11-13,15,19H,7-10,16H2,1-4H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Abl kinase |
J Med Chem 47: 1599-601 (2004)
Article DOI: 10.1021/jm0499458
BindingDB Entry DOI: 10.7270/Q2BP05HD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
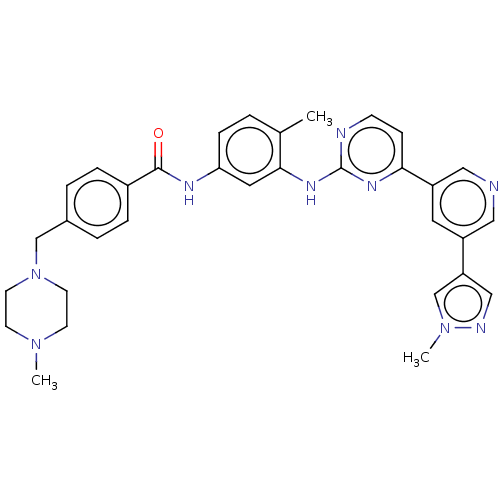
(Homo sapiens (Human)) | BDBM612893
(US11725005, Compound 8300)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cncc(c3)-c3cnn(C)c3)c2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R215H9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
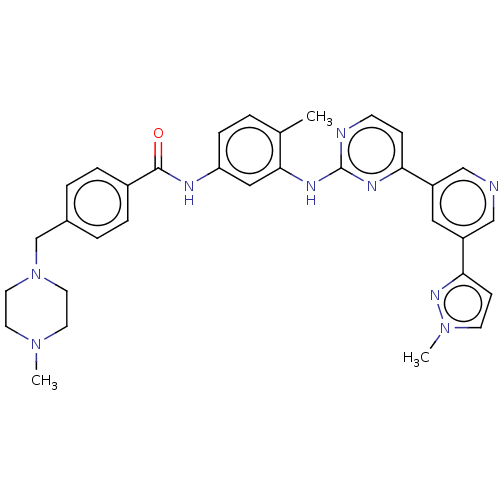
(Homo sapiens (Human)) | BDBM481131
(US10906896, Cpd 8300 | US10906896, Cpd 832 | US117...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cncc(c3)-c3ccn(C)n3)c2)CC1 Show InChI InChI=1S/C33H35N9O/c1-23-4-9-28(36-32(43)25-7-5-24(6-8-25)22-42-16-14-40(2)15-17-42)19-31(23)38-33-35-12-10-29(37-33)26-18-27(21-34-20-26)30-11-13-41(3)39-30/h4-13,18-21H,14-17,22H2,1-3H3,(H,36,43)(H,35,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Inhibikase Therapeutics, Inc.
US Patent
| Assay Description
Kinase base buffer (50 mM HEPES, pH 7.5 0.0015% Brij-35; 10 mM MgCl2 2 mM DTT) and Stop buffer, (100 mM HEPES, pH 7.5 0.015% Brij-35; 0.2% Coating Re... |
US Patent US10906896 (2021)
BindingDB Entry DOI: 10.7270/Q2G44TCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427742

(CHEMBL2324930)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(c2)C#Cc2cnc3cccnn23)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-9-24(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)35-28(39)22-6-7-23(26(17-22)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
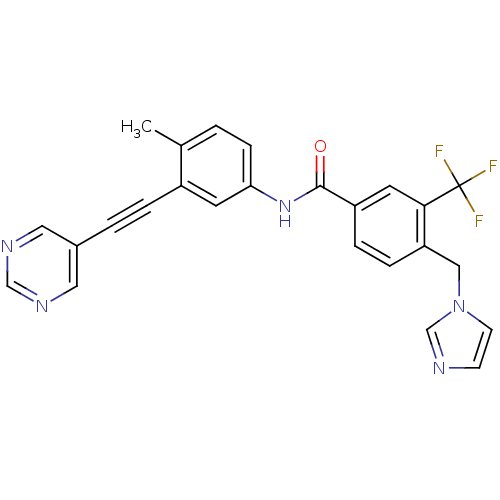
(Homo sapiens (Human)) | BDBM50427740
(CHEMBL2324932)Show SMILES Cc1ccc(NC(=O)c2ccc(Cn3ccnc3)c(c2)C(F)(F)F)cc1C#Cc1cncnc1 Show InChI InChI=1S/C25H18F3N5O/c1-17-2-7-22(10-19(17)4-3-18-12-30-15-31-13-18)32-24(34)20-5-6-21(14-33-9-8-29-16-33)23(11-20)25(26,27)28/h2,5-13,15-16H,14H2,1H3,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM50097947
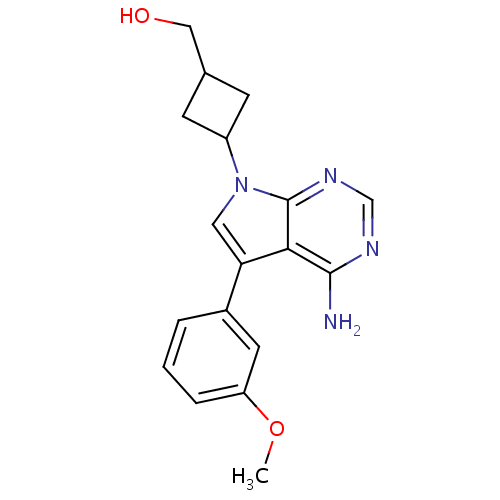
(CHEMBL352801 | {3-[4-Amino-5-(3-methoxy-phenyl)-py...)Show InChI InChI=1S/C18H20N4O2/c1-24-14-4-2-3-12(7-14)15-8-22(13-5-11(6-13)9-23)18-16(15)17(19)20-10-21-18/h2-4,7-8,10-11,13,23H,5-6,9H2,1H3,(H2,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research
Curated by ChEMBL
| Assay Description
Inhibition of v-Abl tyrosine kinase activity |
Bioorg Med Chem Lett 11: 849-52 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RMF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM50097937

(3-(4-Amino-5-phenyl-pyrrolo[2,3-d]pyrimidin-7-yl)-...)Show InChI InChI=1S/C17H18N4O/c18-16-15-14(11-4-2-1-3-5-11)9-21(17(15)20-10-19-16)12-6-7-13(22)8-12/h1-5,9-10,12-13,22H,6-8H2,(H2,18,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research
Curated by ChEMBL
| Assay Description
Inhibition of v-Abl tyrosine kinase activity |
Bioorg Med Chem Lett 11: 849-52 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RMF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University/Royal Victoria Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Abl tyrosine kinase |
Bioorg Med Chem Lett 13: 3297-300 (2003)
BindingDB Entry DOI: 10.7270/Q22B8XFQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427741

(CHEMBL2324931)Show SMILES Cc1ccc(NC(=O)c2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)cc1C#Cc1cnc2ccccn12 Show InChI InChI=1S/C31H30F3N5O2/c1-22-5-9-26(18-23(22)8-10-27-20-35-29-4-2-3-11-39(27)29)36-30(41)24-6-7-25(28(19-24)31(32,33)34)21-38-14-12-37(13-15-38)16-17-40/h2-7,9,11,18-20,40H,12-17,21H2,1H3,(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM612785
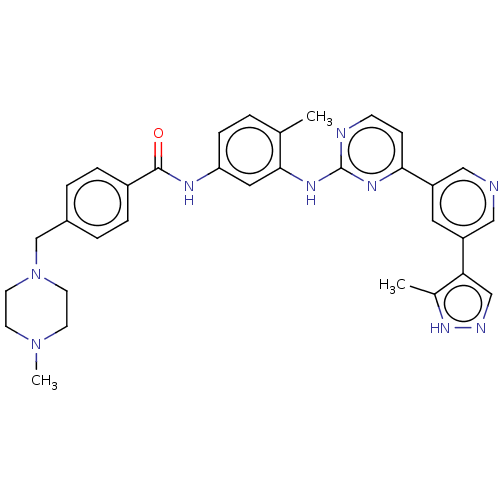
(US11725005, Compound 119)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cncc(c3)-c3cn[nH]c3C)c2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 44.7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R215H9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM481115
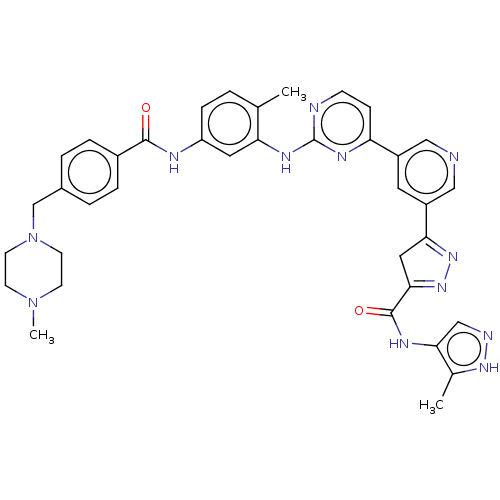
(US10906896, Cpd 119)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cncc(c3)C3=NN=C(C3)C(=O)Nc3cn[nH]c3C)c2)CC1 |c:39,t:37| Show InChI InChI=1S/C37H38N12O2/c1-23-4-9-29(41-35(50)26-7-5-25(6-8-26)22-49-14-12-48(3)13-15-49)17-31(23)44-37-39-11-10-30(43-37)27-16-28(20-38-19-27)32-18-33(47-46-32)36(51)42-34-21-40-45-24(34)2/h4-11,16-17,19-21H,12-15,18,22H2,1-3H3,(H,40,45)(H,41,50)(H,42,51)(H,39,43,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 44.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Inhibikase Therapeutics, Inc.
US Patent
| Assay Description
Kinase base buffer (50 mM HEPES, pH 7.5 0.0015% Brij-35; 10 mM MgCl2 2 mM DTT) and Stop buffer, (100 mM HEPES, pH 7.5 0.015% Brij-35; 0.2% Coating Re... |
US Patent US10906896 (2021)
BindingDB Entry DOI: 10.7270/Q2G44TCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50427747

(CHEMBL2324926)Show SMILES CN1CCN(Cc2ccc(NC(=O)Nc3cc(C#Cc4cnc5cccnn45)n(C)n3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H26F3N9O/c1-35-10-12-37(13-11-35)17-18-5-6-19(14-22(18)26(27,28)29)32-25(39)33-23-15-20(36(2)34-23)7-8-21-16-30-24-4-3-9-31-38(21)24/h3-6,9,14-16H,10-13,17H2,1-2H3,(H2,32,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclofluidic Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Abl2 kinase (unknown origin) using Tyr 6 peptide as substrate assessed as residual enzyme activity after every 10 secs measured for 10 ... |
J Med Chem 56: 3033-47 (2013)
Article DOI: 10.1021/jm400099d
BindingDB Entry DOI: 10.7270/Q24T6KPD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM50097945
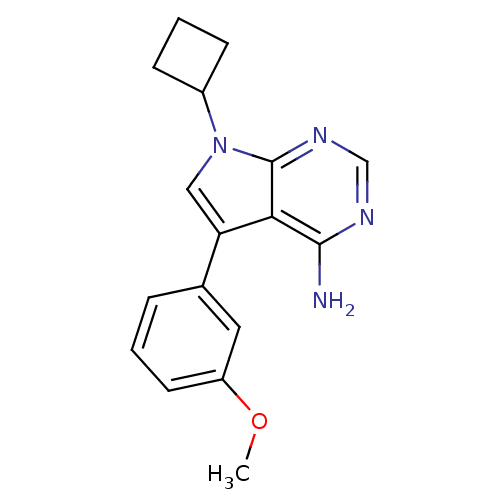
(7-Cyclobutyl-5-(3-methoxy-phenyl)-7H-pyrrolo[2,3-d...)Show InChI InChI=1S/C17H18N4O/c1-22-13-7-2-4-11(8-13)14-9-21(12-5-3-6-12)17-15(14)16(18)19-10-20-17/h2,4,7-10,12H,3,5-6H2,1H3,(H2,18,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research
Curated by ChEMBL
| Assay Description
Inhibition of p60 c-Src tyrosine kinase mediated phosphorylation of Fak in IC8.1 fibroblasts |
Bioorg Med Chem Lett 11: 849-52 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RMF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1/ABL2
(Homo sapiens (Human)) | BDBM50088904

((3-(4-amino-5-(3-methoxyphenyl)-7H-pyrrolo[2,3-d]p...)Show SMILES COc1cccc(c1)-c1cn(-c2cccc(CO)c2)c2ncnc(N)c12 Show InChI InChI=1S/C20H18N4O2/c1-26-16-7-3-5-14(9-16)17-10-24(15-6-2-4-13(8-15)11-25)20-18(17)19(21)22-12-23-20/h2-10,12,25H,11H2,1H3,(H2,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of non-receptor tyrosine kinase v-Abl |
Bioorg Med Chem Lett 10: 945-9 (2000)
BindingDB Entry DOI: 10.7270/Q2R78DG4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM481109
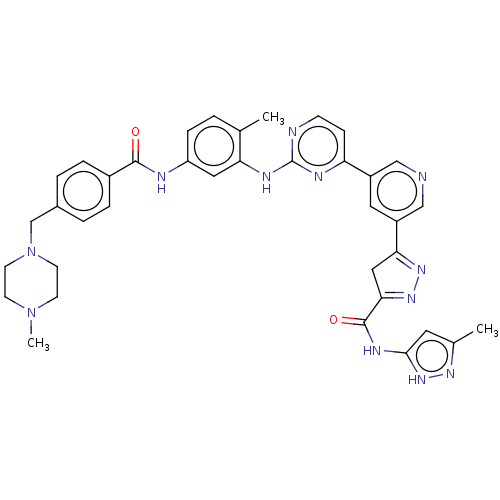
(US10906896, Cpd 113)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cncc(c3)C3=NN=C(C3)C(=O)Nc3cc(C)n[nH]3)c2)CC1 |c:39,t:37| Show InChI InChI=1S/C37H38N12O2/c1-23-4-9-29(40-35(50)26-7-5-25(6-8-26)22-49-14-12-48(3)13-15-49)18-31(23)42-37-39-11-10-30(41-37)27-17-28(21-38-20-27)32-19-33(46-45-32)36(51)43-34-16-24(2)44-47-34/h4-11,16-18,20-21H,12-15,19,22H2,1-3H3,(H,40,50)(H,39,41,42)(H2,43,44,47,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Inhibikase Therapeutics, Inc.
US Patent
| Assay Description
Kinase base buffer (50 mM HEPES, pH 7.5 0.0015% Brij-35; 10 mM MgCl2 2 mM DTT) and Stop buffer, (100 mM HEPES, pH 7.5 0.015% Brij-35; 0.2% Coating Re... |
US Patent US10906896 (2021)
BindingDB Entry DOI: 10.7270/Q2G44TCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM612772
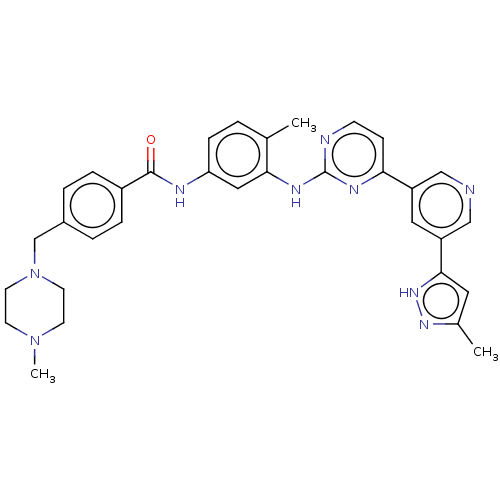
(US11725005, Compound 113)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cncc(c3)-c3cc(C)n[nH]3)c2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R215H9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data