 Found 2220 hits for UniProtKB: P49354
Found 2220 hits for UniProtKB: P49354 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50287709

((E)-3-Carboxy-2-(16-sulfooxy-hexadecyl)-pent-2-ene...)Show SMILES OC(=O)C\C(C(O)=O)=C(\CCCCCCCCCCCCCCCCOS(O)(=O)=O)C(O)=O Show InChI InChI=1S/C22H38O10S/c23-20(24)17-19(22(27)28)18(21(25)26)15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16-32-33(29,30)31/h1-17H2,(H,23,24)(H,25,26)(H,27,28)(H,29,30,31)/b19-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant Protein farnesyltransferase with respect to FPP |
Bioorg Med Chem Lett 6: 2081-2084 (1996)
Article DOI: 10.1016/0960-894X(96)00372-1
BindingDB Entry DOI: 10.7270/Q2222TQ1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50492066
(CHEMBL2396761)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CS)NC(=O)CCCCCNC(=O)c1cc(OCCC(CN)CN)c(OCCC(CN)CN)c(OCCC(CN)CN)c1)C(C)C)C(=O)N[C@@H](CCSC)C(O)=O |r| Show InChI InChI=1S/C47H87N11O10S2/c1-6-30(4)41(46(63)56-35(47(64)65)14-19-70-5)58-45(62)40(29(2)3)57-44(61)36(28-69)55-39(59)10-8-7-9-15-54-43(60)34-20-37(66-16-11-31(22-48)23-49)42(68-18-13-33(26-52)27-53)38(21-34)67-17-12-32(24-50)25-51/h20-21,29-33,35-36,40-41,69H,6-19,22-28,48-53H2,1-5H3,(H,54,60)(H,55,59)(H,56,63)(H,57,61)(H,58,62)(H,64,65)/t30-,35-,36-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of FTase (unknown origin)-mediated K-Ras4B model peptide KKKKKKSK(Dans)TKCVIM farnesylation by microplate reader analysis |
Bioorg Med Chem 21: 4004-10 (2013)
Article DOI: 10.1016/j.bmc.2012.09.061
BindingDB Entry DOI: 10.7270/Q2ZS30GN |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50059860
(((2E,6E)-1-Hydroxy-3,7,11-trimethyl-dodeca-2,6,10-...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6](=O)P([#8])([#8])=O Show InChI InChI=1S/C15H27O4P/c1-12(2)7-5-8-13(3)9-6-10-14(4)11-15(16)20(17,18)19/h7,9,14H,5-6,8,10-11H2,1-4H3,(H2,17,18,19)/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against farnesyl pyrophosphate |
J Med Chem 40: 2971-90 (1997)
Article DOI: 10.1021/jm970226l
BindingDB Entry DOI: 10.7270/Q26M37HF |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50143597
((S)-2-(2-[2-(4-Fluoro-phenyl)-ethyl]-5-{[(1S,4S)-4...)Show SMILES CSCC[C@H](NC(=O)c1cc(NC[C@@H]2C[C@@H](CN2)SC(=O)c2cccnc2)ccc1CCc1ccc(F)cc1)C(=O)OC(C)C Show InChI InChI=1S/C34H41FN4O4S2/c1-22(2)43-33(41)31(14-16-44-3)39-32(40)30-18-27(13-10-24(30)9-6-23-7-11-26(35)12-8-23)37-20-28-17-29(21-38-28)45-34(42)25-5-4-15-36-19-25/h4-5,7-8,10-13,15,18-19,22,28-29,31,37-38H,6,9,14,16-17,20-21H2,1-3H3,(H,39,40)/t28-,29-,31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition binding constant against Geranylgeranyl transferase type I |
J Med Chem 47: 1869-78 (2004)
Article DOI: 10.1021/jm0305467
BindingDB Entry DOI: 10.7270/Q2RX9CVX |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50492066

(CHEMBL2396761)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CS)NC(=O)CCCCCNC(=O)c1cc(OCCC(CN)CN)c(OCCC(CN)CN)c(OCCC(CN)CN)c1)C(C)C)C(=O)N[C@@H](CCSC)C(O)=O |r| Show InChI InChI=1S/C47H87N11O10S2/c1-6-30(4)41(46(63)56-35(47(64)65)14-19-70-5)58-45(62)40(29(2)3)57-44(61)36(28-69)55-39(59)10-8-7-9-15-54-43(60)34-20-37(66-16-11-31(22-48)23-49)42(68-18-13-33(26-52)27-53)38(21-34)67-17-12-32(24-50)25-51/h20-21,29-33,35-36,40-41,69H,6-19,22-28,48-53H2,1-5H3,(H,54,60)(H,55,59)(H,56,63)(H,57,61)(H,58,62)(H,64,65)/t30-,35-,36-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FTase (unknown origin)-mediated K-Ras4B model peptide KKKKKKSK(Dans)TKCVIM farnesylation by fluorescence plate reader analy... |
Bioorg Med Chem 21: 4004-10 (2013)
Article DOI: 10.1016/j.bmc.2012.09.061
BindingDB Entry DOI: 10.7270/Q2ZS30GN |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50289150

(Acetic acid (7E,11E)-(1S,2R,4R,14S,15R)-4,8,12-tri...)Show SMILES CC(=O)O[C@H]1C\C(C)=C\CC\C(C)=C\CC[C@@]2(C)O[C@@H]2[C@H]2OC(=O)C(=C)[C@H]12 |t:7,12| Show InChI InChI=1S/C22H30O5/c1-13-8-6-9-14(2)12-17(25-16(4)23)18-15(3)21(24)26-19(18)20-22(5,27-20)11-7-10-13/h9-10,17-20H,3,6-8,11-12H2,1-2,4-5H3/b13-10+,14-9+/t17-,18+,19-,20+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Kinetic parameter for inihibiton of farnesyl protein transferase |
Bioorg Med Chem Lett 6: 909-912 (1996)
Article DOI: 10.1016/0960-894X(96)00142-4
BindingDB Entry DOI: 10.7270/Q2VT1S3W |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50481616
(CHEMBL590127)Show SMILES [#6]-[#6]-[#8]-[#6](=O)C([#6]-[#6]-[#6]-c1ncc(-[#6]-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](-[#8])=O)n1-[#6])([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6](-[#8])=O |r| Show InChI InChI=1S/C47H71N5O8S/c1-10-60-46(59)47(45(57)58,27-24-35(7)20-15-19-34(6)18-14-17-32(2)3)26-16-23-40-48-30-37(52(40)8)31-49-41(33(4)5)43(54)51-39(29-36-21-12-11-13-22-36)42(53)50-38(44(55)56)25-28-61-9/h11-13,17,19,21-22,24,30,33,38-39,41,49H,10,14-16,18,20,23,25-29,31H2,1-9H3,(H,50,53)(H,51,54)(H,55,56)(H,57,58)/b34-19+,35-24+/t38-,39-,41-,47?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette
Curated by ChEMBL
| Assay Description
Binding affinity to FPP site of human recombinant FTase by competitive Michaelis-Menten analysis |
Bioorg Med Chem 18: 543-56 (2010)
Article DOI: 10.1016/j.bmc.2009.12.017
BindingDB Entry DOI: 10.7270/Q2VM4G37 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50481625

(CHEMBL599795)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6](-[#6](-[#8])=O)-[#6](-[#8])=O Show InChI InChI=1S/C18H28O4/c1-13(2)7-5-8-14(3)9-6-10-15(4)11-12-16(17(19)20)18(21)22/h7,9,11,16H,5-6,8,10,12H2,1-4H3,(H,19,20)(H,21,22)/b14-9+,15-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette
Curated by ChEMBL
| Assay Description
Binding affinity to FPP site of human recombinant FTase by competitive Michaelis-Menten analysis |
Bioorg Med Chem 18: 543-56 (2010)
Article DOI: 10.1016/j.bmc.2009.12.017
BindingDB Entry DOI: 10.7270/Q2VM4G37 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50481615

(CHEMBL590126)Show SMILES [#6]-[#6]-[#8]-[#6](=O)C([#6]\[#6]=[#6]\c1ncc(-[#6]-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](-[#8])=O)n1-[#6])([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6](-[#8])=O |r| Show InChI InChI=1S/C47H69N5O8S/c1-10-60-46(59)47(45(57)58,27-24-35(7)20-15-19-34(6)18-14-17-32(2)3)26-16-23-40-48-30-37(52(40)8)31-49-41(33(4)5)43(54)51-39(29-36-21-12-11-13-22-36)42(53)50-38(44(55)56)25-28-61-9/h11-13,16-17,19,21-24,30,33,38-39,41,49H,10,14-15,18,20,25-29,31H2,1-9H3,(H,50,53)(H,51,54)(H,55,56)(H,57,58)/b23-16+,34-19+,35-24+/t38-,39-,41-,47?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette
Curated by ChEMBL
| Assay Description
Binding affinity to FPP site of human recombinant FTase by competitive Michaelis-Menten analysis |
Bioorg Med Chem 18: 543-56 (2010)
Article DOI: 10.1016/j.bmc.2009.12.017
BindingDB Entry DOI: 10.7270/Q2VM4G37 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50108070

(2-[4-(2-Amino-3-mercapto-propylamino)-benzoylamino...)Show SMILES CSCC[C@H](NC(=O)c1ccc(NC[C@@H](N)CS)cc1)C(O)=O Show InChI InChI=1S/C15H23N3O3S2/c1-23-7-6-13(15(20)21)18-14(19)10-2-4-12(5-3-10)17-8-11(16)9-22/h2-5,11,13,17,22H,6-9,16H2,1H3,(H,18,19)(H,20,21)/t11-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 785 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FTase (unknown origin)-mediated K-Ras4B model peptide KKKKKKSK(Dans)TKCVIM farnesylation by fluorescence plate reader analy... |
Bioorg Med Chem 21: 4004-10 (2013)
Article DOI: 10.1016/j.bmc.2012.09.061
BindingDB Entry DOI: 10.7270/Q2ZS30GN |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50059865
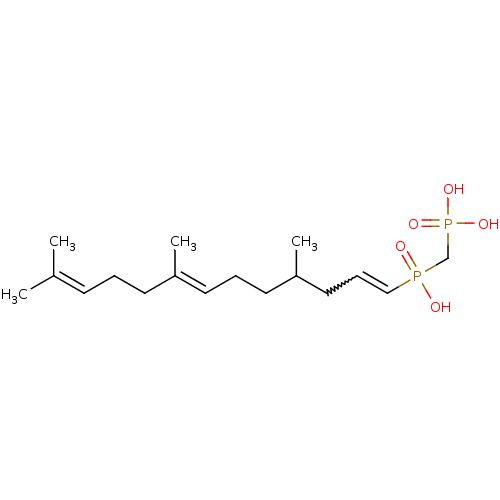
(4,8,12-trimethyl-(3E,7E)-3,7,11-tridecatrienylhydr...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6]=[#6]P([#8])(=O)[#6]P([#8])([#8])=O |w:14.13| Show InChI InChI=1S/C17H32O5P2/c1-15(2)8-5-9-16(3)10-6-11-17(4)12-7-13-23(18,19)14-24(20,21)22/h7-8,10,13,17H,5-6,9,11-12,14H2,1-4H3,(H,18,19)(H2,20,21,22)/b13-7?,16-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against farnesyl pyrophosphate |
J Med Chem 40: 2971-90 (1997)
Article DOI: 10.1021/jm970226l
BindingDB Entry DOI: 10.7270/Q26M37HF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50492065
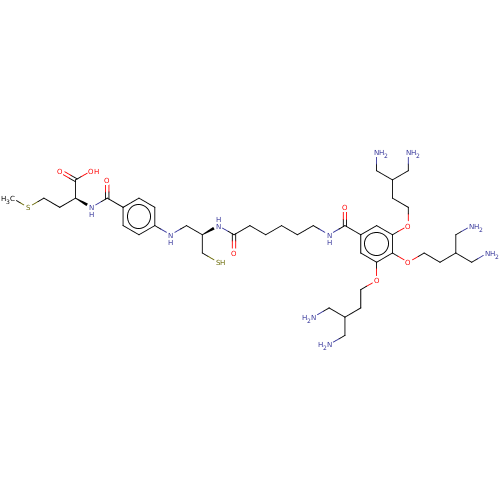
(CHEMBL2396762)Show SMILES CSCC[C@H](NC(=O)c1ccc(NC[C@H](CS)NC(=O)CCCCCNC(=O)c2cc(OCCC(CN)CN)c(OCCC(CN)CN)c(OCCC(CN)CN)c2)cc1)C(O)=O |r| Show InChI InChI=1S/C43H74N10O8S2/c1-63-18-13-36(43(57)58)53-42(56)32-6-8-34(9-7-32)51-27-35(28-62)52-39(54)5-3-2-4-14-50-41(55)33-19-37(59-15-10-29(21-44)22-45)40(61-17-12-31(25-48)26-49)38(20-33)60-16-11-30(23-46)24-47/h6-9,19-20,29-31,35-36,51,62H,2-5,10-18,21-28,44-49H2,1H3,(H,50,55)(H,52,54)(H,53,56)(H,57,58)/t35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 837 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FTase (unknown origin)-mediated K-Ras4B model peptide KKKKKKSK(Dans)TKCVIM farnesylation by fluorescence plate reader analy... |
Bioorg Med Chem 21: 4004-10 (2013)
Article DOI: 10.1016/j.bmc.2012.09.061
BindingDB Entry DOI: 10.7270/Q2ZS30GN |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50108076
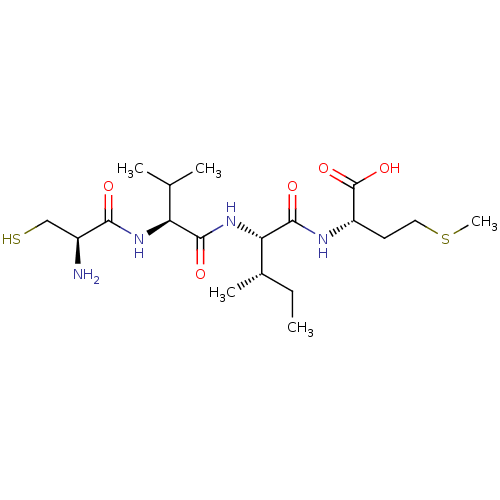
((S)-2-{(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propio...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C)C(=O)N[C@@H](CCSC)C(O)=O Show InChI InChI=1S/C19H36N4O5S2/c1-6-11(4)15(18(26)21-13(19(27)28)7-8-30-5)23-17(25)14(10(2)3)22-16(24)12(20)9-29/h10-15,29H,6-9,20H2,1-5H3,(H,21,26)(H,22,24)(H,23,25)(H,27,28)/t11-,12-,13-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of FTase (unknown origin)-mediated K-Ras4B model peptide KKKKKKSK(Dans)TKCVIM farnesylation by microplate reader analysis |
Bioorg Med Chem 21: 4004-10 (2013)
Article DOI: 10.1016/j.bmc.2012.09.061
BindingDB Entry DOI: 10.7270/Q2ZS30GN |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50481616

(CHEMBL590127)Show SMILES [#6]-[#6]-[#8]-[#6](=O)C([#6]-[#6]-[#6]-c1ncc(-[#6]-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](-[#8])=O)n1-[#6])([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6](-[#8])=O |r| Show InChI InChI=1S/C47H71N5O8S/c1-10-60-46(59)47(45(57)58,27-24-35(7)20-15-19-34(6)18-14-17-32(2)3)26-16-23-40-48-30-37(52(40)8)31-49-41(33(4)5)43(54)51-39(29-36-21-12-11-13-22-36)42(53)50-38(44(55)56)25-28-61-9/h11-13,17,19,21-22,24,30,33,38-39,41,49H,10,14-16,18,20,23,25-29,31H2,1-9H3,(H,50,53)(H,51,54)(H,55,56)(H,57,58)/b34-19+,35-24+/t38-,39-,41-,47?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette
Curated by ChEMBL
| Assay Description
Binding affinity to CaaX site of human recombinant FTase by non-competitive Michaelis-Menten analysis for enzyme-substrate-inhibitor complex |
Bioorg Med Chem 18: 543-56 (2010)
Article DOI: 10.1016/j.bmc.2009.12.017
BindingDB Entry DOI: 10.7270/Q2VM4G37 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50481616

(CHEMBL590127)Show SMILES [#6]-[#6]-[#8]-[#6](=O)C([#6]-[#6]-[#6]-c1ncc(-[#6]-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](-[#8])=O)n1-[#6])([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6](-[#8])=O |r| Show InChI InChI=1S/C47H71N5O8S/c1-10-60-46(59)47(45(57)58,27-24-35(7)20-15-19-34(6)18-14-17-32(2)3)26-16-23-40-48-30-37(52(40)8)31-49-41(33(4)5)43(54)51-39(29-36-21-12-11-13-22-36)42(53)50-38(44(55)56)25-28-61-9/h11-13,17,19,21-22,24,30,33,38-39,41,49H,10,14-16,18,20,23,25-29,31H2,1-9H3,(H,50,53)(H,51,54)(H,55,56)(H,57,58)/b34-19+,35-24+/t38-,39-,41-,47?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette
Curated by ChEMBL
| Assay Description
Binding affinity to CaaX site of human recombinant FTase by non-competitive Michaelis-Menten analysis for enzyme-inhibitor complex |
Bioorg Med Chem 18: 543-56 (2010)
Article DOI: 10.1016/j.bmc.2009.12.017
BindingDB Entry DOI: 10.7270/Q2VM4G37 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50481615

(CHEMBL590126)Show SMILES [#6]-[#6]-[#8]-[#6](=O)C([#6]\[#6]=[#6]\c1ncc(-[#6]-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](-[#8])=O)n1-[#6])([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6](-[#8])=O |r| Show InChI InChI=1S/C47H69N5O8S/c1-10-60-46(59)47(45(57)58,27-24-35(7)20-15-19-34(6)18-14-17-32(2)3)26-16-23-40-48-30-37(52(40)8)31-49-41(33(4)5)43(54)51-39(29-36-21-12-11-13-22-36)42(53)50-38(44(55)56)25-28-61-9/h11-13,16-17,19,21-24,30,33,38-39,41,49H,10,14-15,18,20,25-29,31H2,1-9H3,(H,50,53)(H,51,54)(H,55,56)(H,57,58)/b23-16+,34-19+,35-24+/t38-,39-,41-,47?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette
Curated by ChEMBL
| Assay Description
Binding affinity to CaaX site of human recombinant FTase by non-competitive Michaelis-Menten analysis for enzyme-substrate-inhibitor complex |
Bioorg Med Chem 18: 543-56 (2010)
Article DOI: 10.1016/j.bmc.2009.12.017
BindingDB Entry DOI: 10.7270/Q2VM4G37 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50481615

(CHEMBL590126)Show SMILES [#6]-[#6]-[#8]-[#6](=O)C([#6]\[#6]=[#6]\c1ncc(-[#6]-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](-[#8])=O)n1-[#6])([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6](-[#8])=O |r| Show InChI InChI=1S/C47H69N5O8S/c1-10-60-46(59)47(45(57)58,27-24-35(7)20-15-19-34(6)18-14-17-32(2)3)26-16-23-40-48-30-37(52(40)8)31-49-41(33(4)5)43(54)51-39(29-36-21-12-11-13-22-36)42(53)50-38(44(55)56)25-28-61-9/h11-13,16-17,19,21-24,30,33,38-39,41,49H,10,14-15,18,20,25-29,31H2,1-9H3,(H,50,53)(H,51,54)(H,55,56)(H,57,58)/b23-16+,34-19+,35-24+/t38-,39-,41-,47?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette
Curated by ChEMBL
| Assay Description
Binding affinity to CaaX site of human recombinant FTase by non-competitive Michaelis-Menten analysis for enzyme-inhibitor complex |
Bioorg Med Chem 18: 543-56 (2010)
Article DOI: 10.1016/j.bmc.2009.12.017
BindingDB Entry DOI: 10.7270/Q2VM4G37 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14023

((1R,2R,5R)-30-oxo-19-oxa-2,6,10,12-tetraazahexacyc...)Show SMILES O=C1[C@H]2CCN1[C@@H]1CCCc3ccc(Oc4cc(Cn5cncc5CN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C26H25N5O2/c27-12-19-5-4-17-10-25(19)33-21-7-6-18-2-1-3-24(22(18)11-21)31-9-8-23(26(31)32)29-14-20-13-28-16-30(20)15-17/h4-7,10-11,13,16,23-24,29H,1-3,8-9,14-15H2/t23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FTase using [3H]farnesyldiphosphate |
Medchemcomm 4: 476-492 (2013)
Article DOI: 10.1039/c2md20299a
BindingDB Entry DOI: 10.7270/Q2HD7ZN5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50135360
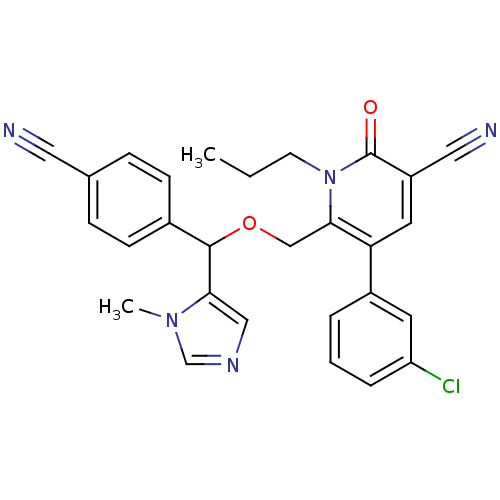
(5-(3-Chloro-phenyl)-6-[(4-cyano-phenyl)-(3-methyl-...)Show SMILES CCCn1c(COC(c2cncn2C)c2ccc(cc2)C#N)c(cc(C#N)c1=O)-c1cccc(Cl)c1 Show InChI InChI=1S/C28H24ClN5O2/c1-3-11-34-26(24(13-22(15-31)28(34)35)21-5-4-6-23(29)12-21)17-36-27(25-16-32-18-33(25)2)20-9-7-19(14-30)8-10-20/h4-10,12-13,16,18,27H,3,11,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against farnesyltransferase (FT) |
Bioorg Med Chem Lett 13: 4001-5 (2003)
BindingDB Entry DOI: 10.7270/Q2H41QVX |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50520989
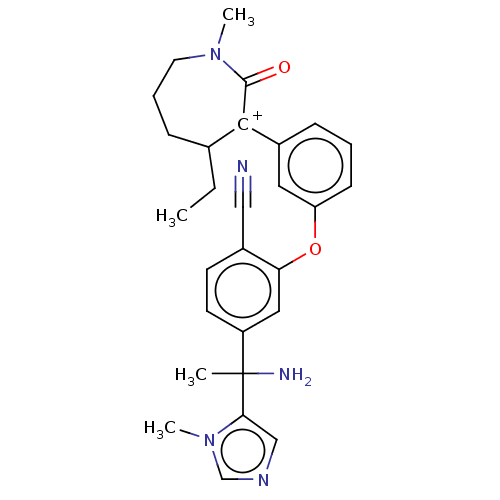
(CHEMBL4565280)Show SMILES CCC1CCCN(C)C(=O)[C+]1c1cccc(Oc2cc(ccc2C#N)C(C)(N)c2cncn2C)c1 Show InChI InChI=1S/C28H32N5O2/c1-5-19-9-7-13-32(3)27(34)26(19)20-8-6-10-23(14-20)35-24-15-22(12-11-21(24)16-29)28(2,30)25-17-31-18-33(25)4/h6,8,10-12,14-15,17-19H,5,7,9,13,30H2,1-4H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FTase using [3H]FPP as substrate after 15 mins by scintillation counting analysis |
Eur J Med Chem 162: 465-494 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.031
BindingDB Entry DOI: 10.7270/Q2154MGW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50126028

(3',5'-Dichloro-6-[3-(4-cyano-phenyl)-3-hydroxy-3-(...)Show SMILES Cn1cncc1C(O)(C#Cc1ccc(cc1-c1cc(Cl)cc(Cl)c1)C#N)c1ccc(cc1)C#N Show InChI InChI=1S/C27H16Cl2N4O/c1-33-17-32-16-26(33)27(34,22-6-3-18(14-30)4-7-22)9-8-20-5-2-19(15-31)10-25(20)21-11-23(28)13-24(29)12-21/h2-7,10-13,16-17,34H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human FTase-catalyzed incorporation of [3H]-FPP into recombinant Ras CVIM |
Bioorg Med Chem Lett 13: 1293-6 (2003)
BindingDB Entry DOI: 10.7270/Q2DV1J8J |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50101929
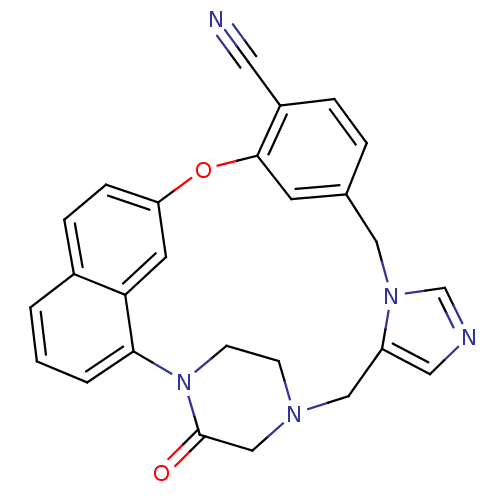
(3-oxo-18-oxa-2,5,9,11-tetraazahexacyclo[17.6.2.22,...)Show SMILES O=C1CN2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5C2)ccc4C#N)cc13 Show InChI InChI=1S/C26H21N5O2/c27-12-20-5-4-18-10-25(20)33-22-7-6-19-2-1-3-24(23(19)11-22)31-9-8-29(16-26(31)32)15-21-13-28-17-30(21)14-18/h1-7,10-11,13,17H,8-9,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM |
Bioorg Med Chem Lett 11: 1817-21 (2001)
BindingDB Entry DOI: 10.7270/Q2R210PG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139186
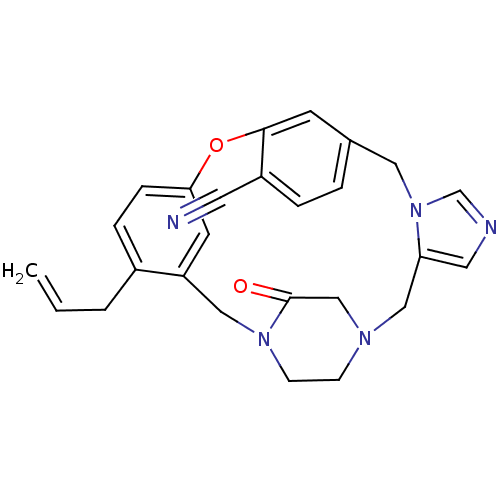
(4-allyl-23-oxo-8-oxa-1,15,17,21-tetraazapentacyclo...)Show SMILES C=CCc1ccc2Oc3cc(Cn4cncc4CN4CCN(Cc1c2)C(=O)C4)ccc3C#N Show InChI InChI=1S/C26H25N5O2/c1-2-3-20-6-7-24-11-22(20)15-30-9-8-29(17-26(30)32)16-23-13-28-18-31(23)14-19-4-5-21(12-27)25(10-19)33-24/h2,4-7,10-11,13,18H,1,3,8-9,14-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50495912
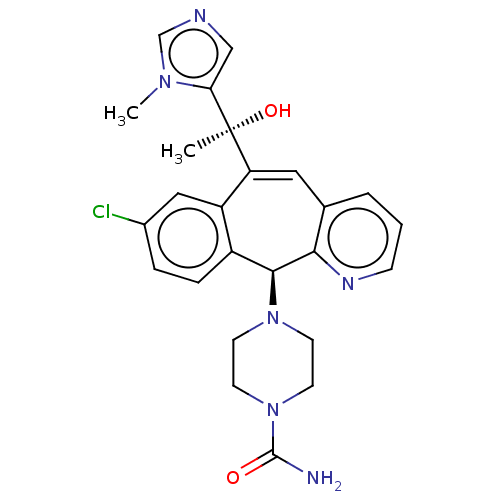
(CHEMBL3115255)Show SMILES Cn1cncc1[C@@](C)(O)C1=Cc2cccnc2[C@@H](N2CCN(CC2)C(N)=O)c2ccc(Cl)cc12 |r,t:10| Show InChI InChI=1S/C25H27ClN6O2/c1-25(34,21-14-28-15-30(21)2)20-12-16-4-3-7-29-22(16)23(18-6-5-17(26)13-19(18)20)31-8-10-32(11-9-31)24(27)33/h3-7,12-15,23,34H,8-11H2,1-2H3,(H2,27,33)/t23-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... |
Bioorg Med Chem Lett 24: 1228-31 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.046
BindingDB Entry DOI: 10.7270/Q26H4MCJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50495899

(CHEMBL3115256)Show SMILES Cn1cncc1[C@@](C)(O)C1=Cc2cccnc2[C@@H](N2CCN(CC2)C(=O)NC2CCCCC2)c2ccc(Cl)cc12 |r,t:10| Show InChI InChI=1S/C31H37ClN6O2/c1-31(40,27-19-33-20-36(27)2)26-17-21-7-6-12-34-28(21)29(24-11-10-22(32)18-25(24)26)37-13-15-38(16-14-37)30(39)35-23-8-4-3-5-9-23/h6-7,10-12,17-20,23,29,40H,3-5,8-9,13-16H2,1-2H3,(H,35,39)/t29-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... |
Bioorg Med Chem Lett 24: 1228-31 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.046
BindingDB Entry DOI: 10.7270/Q26H4MCJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50101929

(3-oxo-18-oxa-2,5,9,11-tetraazahexacyclo[17.6.2.22,...)Show SMILES O=C1CN2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5C2)ccc4C#N)cc13 Show InChI InChI=1S/C26H21N5O2/c27-12-20-5-4-18-10-25(20)33-22-7-6-19-2-1-3-24(23(19)11-22)31-9-8-29(16-26(31)32)15-21-13-28-17-30(21)14-18/h1-7,10-11,13,17H,8-9,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50075841

((S)-2-[(5-{2-[4-(Adamantane-1-carbonyl)-pyridin-3-...)Show SMILES CSCC[C@H](NC(=O)c1ccc(\C=C\c2cnccc2C(=O)[C@]23C[C@H]4C[C@H](C[C@H](C4)C2)C3)cc1-c1ccccc1C)C(O)=O |TLB:25:26:30:23.24.29,THB:25:24:30:31.26.27| Show InChI InChI=1S/C37H40N2O4S/c1-23-5-3-4-6-29(23)32-18-24(8-10-31(32)35(41)39-33(36(42)43)12-14-44-2)7-9-28-22-38-13-11-30(28)34(40)37-19-25-15-26(20-37)17-27(16-25)21-37/h3-11,13,18,22,25-27,33H,12,14-17,19-21H2,1-2H3,(H,39,41)(H,42,43)/b9-7+/t25-,26+,27-,33-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against farnesyltransferase (FT) using SPA assay |
Bioorg Med Chem Lett 9: 703-8 (1999)
BindingDB Entry DOI: 10.7270/Q2G73CWQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50130373

(4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethyl]-2-...)Show SMILES CCCC[C@]1(CCCCN(C)C1=O)c1cccc(Oc2cc(ccc2C#N)[C@](C)(N)c2cncn2C)c1 Show InChI InChI=1S/C30H37N5O2/c1-5-6-14-30(15-7-8-16-34(3)28(30)36)24-10-9-11-25(17-24)37-26-18-23(13-12-22(26)19-31)29(2,32)27-20-33-21-35(27)4/h9-13,17-18,20-21H,5-8,14-16,32H2,1-4H3/t29-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. |
J Med Chem 46: 2973-84 (2003)
Article DOI: 10.1021/jm020587n
BindingDB Entry DOI: 10.7270/Q26Q1Z0P |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079974
((S)-2-[2-({(S)-3-Methyl-2-[2-(3-naphthalen-1-ylmet...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1cccc2ccccc12 Show InChI InChI=1S/C40H47N5O4S/c1-4-28(2)37(43-38(46)21-33-22-41-27-45(33)24-32-16-10-14-30-12-6-8-18-35(30)32)25-44(26-39(47)42-36(40(48)49)19-20-50-3)23-31-15-9-13-29-11-5-7-17-34(29)31/h5-18,22,27-28,36-37H,4,19-21,23-26H2,1-3H3,(H,42,47)(H,43,46)(H,48,49)/t28?,36-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079961
((S)-2-[2-({(S)-3-Methyl-2-[2-(3-naphthalen-2-ylmet...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccc2ccccc2c1 Show InChI InChI=1S/C40H47N5O4S/c1-4-28(2)37(43-38(46)21-34-22-41-27-45(34)23-29-16-17-30-10-5-6-12-32(30)20-29)25-44(26-39(47)42-36(40(48)49)18-19-50-3)24-33-14-9-13-31-11-7-8-15-35(31)33/h5-17,20,22,27-28,36-37H,4,18-19,21,23-26H2,1-3H3,(H,42,47)(H,43,46)(H,48,49)/t28?,36-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50369371
(CHEMBL1790750)Show SMILES CC[C@@H](C)[C@@H](CN(CC(=O)N[C@@H](CCO)C(O)=O)Cc1cccc2ccccc12)NC[C@@H](N)CS Show InChI InChI=1S/C26H40N4O4S/c1-3-18(2)24(28-13-21(27)17-35)15-30(16-25(32)29-23(11-12-31)26(33)34)14-20-9-6-8-19-7-4-5-10-22(19)20/h4-10,18,21,23-24,28,31,35H,3,11-17,27H2,1-2H3,(H,29,32)(H,33,34)/t18-,21-,23+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.123 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant human Ha-Ras by Farnesyltransferase |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50072639
((S)-2-(2-{[(S)-2-((R)-2-Amino-3-mercapto-propylami...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC[C@@H](N)CS Show InChI InChI=1S/C27H42N4O3S2/c1-4-19(2)25(29-14-22(28)18-35)16-31(17-26(32)30-24(27(33)34)12-13-36-3)15-21-10-7-9-20-8-5-6-11-23(20)21/h5-11,19,22,24-25,29,35H,4,12-18,28H2,1-3H3,(H,30,32)(H,33,34)/t19?,22-,24+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.123 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- FPP incorporation into recombinant Ha-Ras by farnesyl transferase at 10 pM |
Bioorg Med Chem Lett 8: 3311-6 (1999)
BindingDB Entry DOI: 10.7270/Q2G44QSV |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50075837
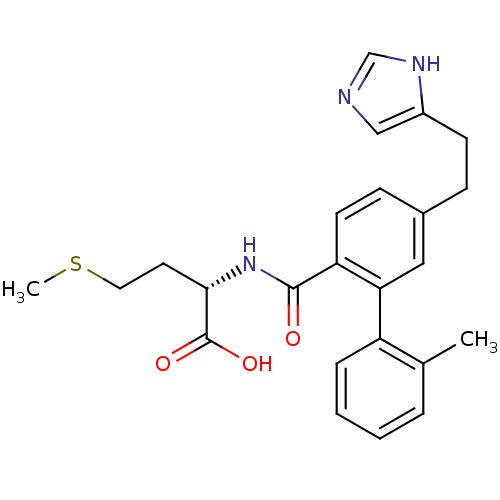
((S)-2-({5-[2-(3H-Imidazol-4-yl)-ethyl]-2'-methyl-b...)Show SMILES CSCC[C@H](NC(=O)c1ccc(CCc2cnc[nH]2)cc1-c1ccccc1C)C(O)=O Show InChI InChI=1S/C24H27N3O3S/c1-16-5-3-4-6-19(16)21-13-17(7-9-18-14-25-15-26-18)8-10-20(21)23(28)27-22(24(29)30)11-12-31-2/h3-6,8,10,13-15,22H,7,9,11-12H2,1-2H3,(H,25,26)(H,27,28)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against farnesyltransferase (FT) using SPA assay |
Bioorg Med Chem Lett 9: 703-8 (1999)
BindingDB Entry DOI: 10.7270/Q2G73CWQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50369443
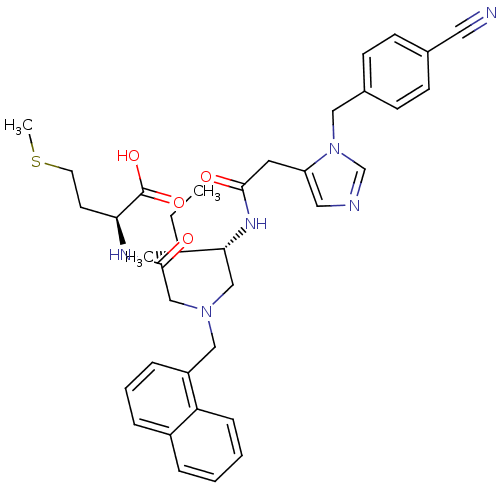
(CHEMBL252953)Show SMILES CC[C@@H](C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccc(cc1)C#N Show InChI InChI=1S/C37H44N6O4S/c1-4-26(2)34(41-35(44)18-31-20-39-25-43(31)21-28-14-12-27(19-38)13-15-28)23-42(24-36(45)40-33(37(46)47)16-17-48-3)22-30-10-7-9-29-8-5-6-11-32(29)30/h5-15,20,25-26,33-34H,4,16-18,21-24H2,1-3H3,(H,40,45)(H,41,44)(H,46,47)/t26-,33+,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079956

((S)-2-[2-({(S)-2-[2-(3-Benzyl-3H-imidazol-4-yl)-ac...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccccc1 Show InChI InChI=1S/C36H45N5O4S/c1-4-26(2)33(39-34(42)19-30-20-37-25-41(30)21-27-11-6-5-7-12-27)23-40(24-35(43)38-32(36(44)45)17-18-46-3)22-29-15-10-14-28-13-8-9-16-31(28)29/h5-16,20,25-26,32-33H,4,17-19,21-24H2,1-3H3,(H,38,43)(H,39,42)(H,44,45)/t26?,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079966
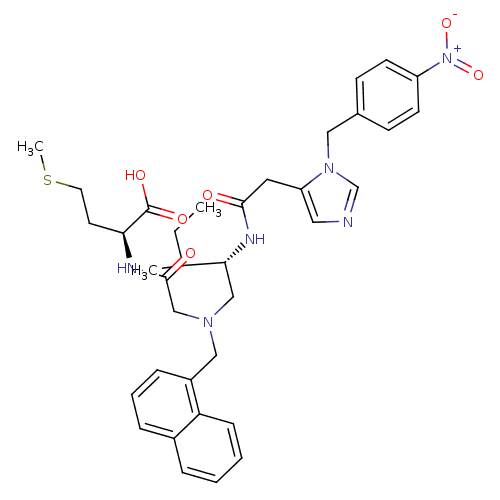
((S)-2-{2-[((S)-3-Methyl-2-{2-[3-(4-nitro-benzyl)-3...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C36H44N6O6S/c1-4-25(2)33(39-34(43)18-30-19-37-24-41(30)20-26-12-14-29(15-13-26)42(47)48)22-40(23-35(44)38-32(36(45)46)16-17-49-3)21-28-10-7-9-27-8-5-6-11-31(27)28/h5-15,19,24-25,32-33H,4,16-18,20-23H2,1-3H3,(H,38,44)(H,39,43)(H,45,46)/t25?,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115916

(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES FC(F)(F)Oc1ccccc1COC(=O)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C25H24F3N5O3/c26-25(27,28)36-23-4-2-1-3-21(23)17-35-24(34)32-11-9-31(10-12-32)16-22-14-30-18-33(22)15-20-7-5-19(13-29)6-8-20/h1-8,14,18H,9-12,15-17H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [1-3H]-GGPP incorporation into biotinylated K4B-Ras peptide by geranylgeranyl transferase in the presence of 5 mM ATP |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50130374
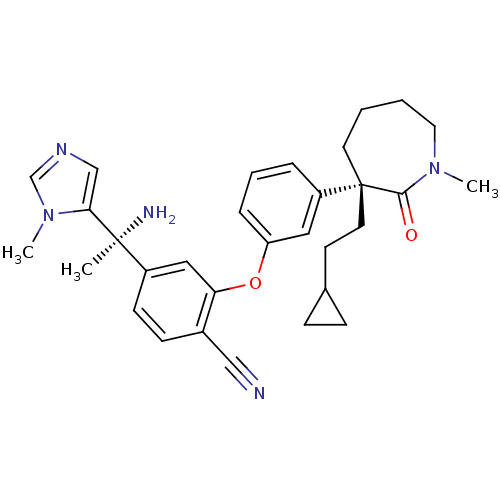
(4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethyl]-2-...)Show SMILES CN1CCCC[C@@](CCC2CC2)(c2cccc(Oc3cc(ccc3C#N)[C@](C)(N)c3cncn3C)c2)C1=O Show InChI InChI=1S/C31H37N5O2/c1-30(33,28-20-34-21-36(28)3)24-12-11-23(19-32)27(18-24)38-26-8-6-7-25(17-26)31(15-13-22-9-10-22)14-4-5-16-35(2)29(31)37/h6-8,11-12,17-18,20-22H,4-5,9-10,13-16,33H2,1-3H3/t30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. |
J Med Chem 46: 2973-84 (2003)
Article DOI: 10.1021/jm020587n
BindingDB Entry DOI: 10.7270/Q26Q1Z0P |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13390

(4-{[(4-cyanophenyl)(1-methyl-1H-imidazol-5-yl)meth...)Show SMILES COCc1cccc(c1)-c1cc(ccc1COC(c1cncn1C)c1ccc(cc1)C#N)C#N Show InChI InChI=1S/C28H24N4O2/c1-32-19-31-16-27(32)28(23-9-6-20(14-29)7-10-23)34-18-25-11-8-21(15-30)13-26(25)24-5-3-4-22(12-24)17-33-2/h3-13,16,19,28H,17-18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories
| Assay Description
The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... |
J Med Chem 47: 612-26 (2004)
Article DOI: 10.1021/jm030434f
BindingDB Entry DOI: 10.7270/Q2Z60M8D |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50130381
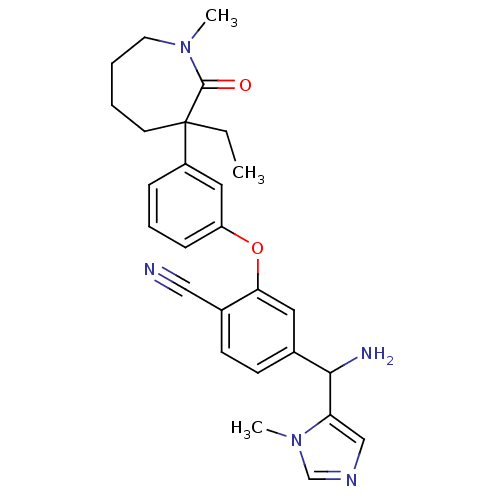
(4-[Amino-(3-methyl-3H-imidazol-4-yl)-methyl]-2-[3-...)Show SMILES CCC1(CCCCN(C)C1=O)c1cccc(Oc2cc(ccc2C#N)C(N)c2cncn2C)c1 Show InChI InChI=1S/C27H31N5O2/c1-4-27(12-5-6-13-31(2)26(27)33)21-8-7-9-22(15-21)34-24-14-19(10-11-20(24)16-28)25(29)23-17-30-18-32(23)3/h7-11,14-15,17-18,25H,4-6,12-13,29H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. |
J Med Chem 46: 2973-84 (2003)
Article DOI: 10.1021/jm020587n
BindingDB Entry DOI: 10.7270/Q26Q1Z0P |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50130365
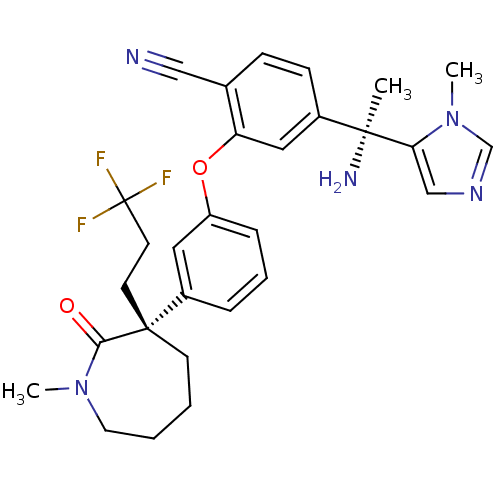
(4-[1-Amino-1-(3-methyl-3H-imidazol-4-yl)-ethyl]-2-...)Show SMILES CN1CCCC[C@@](CCC(F)(F)F)(c2cccc(Oc3cc(ccc3C#N)[C@](C)(N)c3cncn3C)c2)C1=O Show InChI InChI=1S/C29H32F3N5O2/c1-27(34,25-18-35-19-37(25)3)21-10-9-20(17-33)24(16-21)39-23-8-6-7-22(15-23)28(12-13-29(30,31)32)11-4-5-14-36(2)26(28)38/h6-10,15-16,18-19H,4-5,11-14,34H2,1-3H3/t27-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to inhibit recombinant human farnesyltransferase (FTase) catalyzed incorporation of [3H]FPP into recombinant Ras-CVIM. |
J Med Chem 46: 2973-84 (2003)
Article DOI: 10.1021/jm020587n
BindingDB Entry DOI: 10.7270/Q26Q1Z0P |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50098037
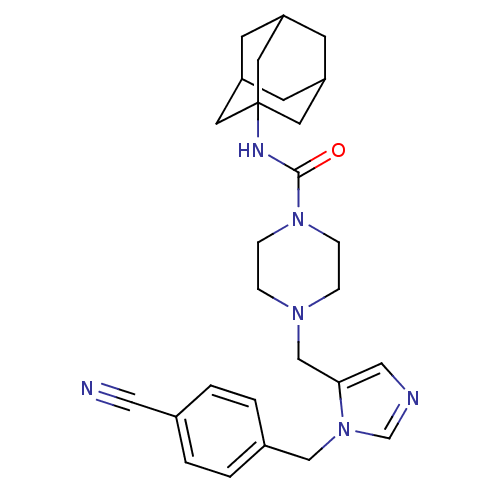
(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES O=C(NC12CC3CC(CC(C3)C1)C2)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 |TLB:10:9:12:5.4.6,THB:8:9:4:7.12.6| Show InChI InChI=1S/C27H34N6O/c28-15-20-1-3-21(4-2-20)17-33-19-29-16-25(33)18-31-5-7-32(8-6-31)26(34)30-27-12-22-9-23(13-27)11-24(10-22)14-27/h1-4,16,19,22-24H,5-14,17-18H2,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Geranylgeranyl transferase type I |
Bioorg Med Chem Lett 11: 865-9 (2001)
BindingDB Entry DOI: 10.7270/Q2F47PPX |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50495906

(CHEMBL3115258)Show SMILES CC(C)OC(=O)N1CCN(CC1)[C@H]1c2ccc(Cl)cc2C(=Cc2cccnc12)[C@](C)(O)c1cncn1C |r,c:22| Show InChI InChI=1S/C28H32ClN5O3/c1-18(2)37-27(35)34-12-10-33(11-13-34)26-21-8-7-20(29)15-22(21)23(14-19-6-5-9-31-25(19)26)28(3,36)24-16-30-17-32(24)4/h5-9,14-18,26,36H,10-13H2,1-4H3/t26-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... |
Bioorg Med Chem Lett 24: 1228-31 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.046
BindingDB Entry DOI: 10.7270/Q26H4MCJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50075851

((S)-2-{[5-(2-Imidazol-1-yl-vinyl)-2'-methyl-biphen...)Show SMILES CSCC[C@H](NC(=O)c1ccc(\C=C\n2ccnc2)cc1-c1ccccc1C)C(O)=O Show InChI InChI=1S/C24H25N3O3S/c1-17-5-3-4-6-19(17)21-15-18(9-12-27-13-11-25-16-27)7-8-20(21)23(28)26-22(24(29)30)10-14-31-2/h3-9,11-13,15-16,22H,10,14H2,1-2H3,(H,26,28)(H,29,30)/b12-9+/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against farnesyltransferase (FT) using SPA assay |
Bioorg Med Chem Lett 9: 703-8 (1999)
BindingDB Entry DOI: 10.7270/Q2G73CWQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50075861
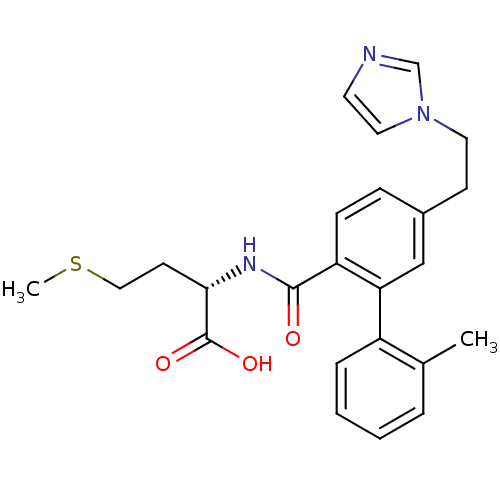
((S)-2-{[5-(2-Imidazol-1-yl-ethyl)-2'-methyl-biphen...)Show SMILES CSCC[C@H](NC(=O)c1ccc(CCn2ccnc2)cc1-c1ccccc1C)C(O)=O Show InChI InChI=1S/C24H27N3O3S/c1-17-5-3-4-6-19(17)21-15-18(9-12-27-13-11-25-16-27)7-8-20(21)23(28)26-22(24(29)30)10-14-31-2/h3-8,11,13,15-16,22H,9-10,12,14H2,1-2H3,(H,26,28)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against farnesyltransferase (FT) using SPA assay |
Bioorg Med Chem Lett 9: 703-8 (1999)
BindingDB Entry DOI: 10.7270/Q2G73CWQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139202
(4-methyl-23-oxo-8-oxa-1,15,17,21-tetraazapentacycl...)Show SMILES Cc1ccc2Oc3cc(Cn4cncc4CN4CCN(Cc1c2)C(=O)C4)ccc3C#N Show InChI InChI=1S/C24H23N5O2/c1-17-2-5-22-9-20(17)13-28-7-6-27(15-24(28)30)14-21-11-26-16-29(21)12-18-3-4-19(10-25)23(8-18)31-22/h2-5,8-9,11,16H,6-7,12-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50126035
(4-[3-(4-Cyano-phenyl)-1-hydroxy-1-(3-methyl-3H-imi...)Show SMILES Cn1cncc1C(O)(C#Cc1ccc(cc1)C#N)c1ccc(C#N)c(c1)-c1cccc2ccccc12 Show InChI InChI=1S/C31H20N4O/c1-35-21-34-20-30(35)31(36,16-15-22-9-11-23(18-32)12-10-22)26-14-13-25(19-33)29(17-26)28-8-4-6-24-5-2-3-7-27(24)28/h2-14,17,20-21,36H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human FTase-catalyzed incorporation of [3H]-FPP into recombinant Ras CVIM |
Bioorg Med Chem Lett 13: 1293-6 (2003)
BindingDB Entry DOI: 10.7270/Q2DV1J8J |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50075844

((S)-2-({5-[2-((E)-4-Hydroxy-pyridin-3-yl)-vinyl]-2...)Show SMILES CSCC[C@H](NC(=O)c1ccc(C=Cc2cnccc2O)cc1-c1ccccc1C)C(O)=O |w:13.13| Show InChI InChI=1S/C26H26N2O4S/c1-17-5-3-4-6-20(17)22-15-18(7-9-19-16-27-13-11-24(19)29)8-10-21(22)25(30)28-23(26(31)32)12-14-33-2/h3-11,13,15-16,23H,12,14H2,1-2H3,(H,27,29)(H,28,30)(H,31,32)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against farnesyltransferase (FT) using SPA assay |
Bioorg Med Chem Lett 9: 703-8 (1999)
BindingDB Entry DOI: 10.7270/Q2G73CWQ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50101929

(3-oxo-18-oxa-2,5,9,11-tetraazahexacyclo[17.6.2.22,...)Show SMILES O=C1CN2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5C2)ccc4C#N)cc13 Show InChI InChI=1S/C26H21N5O2/c27-12-20-5-4-18-10-25(20)33-22-7-6-19-2-1-3-24(23(19)11-22)31-9-8-29(16-26(31)32)15-21-13-28-17-30(21)14-18/h1-7,10-11,13,17H,8-9,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled FTI from Farnesyltransferase in cultured Ha-ras transformed RAT1 cells. |
Bioorg Med Chem Lett 14: 639-43 (2004)
BindingDB Entry DOI: 10.7270/Q2WM1CTK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50075842

((S)-2-({5-[2-((E)-4-Chloro-pyridin-3-yl)-vinyl]-2'...)Show SMILES CSCC[C@H](NC(=O)c1ccc(\C=C\c2cnccc2Cl)cc1-c1ccccc1C)C(O)=O Show InChI InChI=1S/C26H25ClN2O3S/c1-17-5-3-4-6-20(17)22-15-18(7-9-19-16-28-13-11-23(19)27)8-10-21(22)25(30)29-24(26(31)32)12-14-33-2/h3-11,13,15-16,24H,12,14H2,1-2H3,(H,29,30)(H,31,32)/b9-7+/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against farnesyltransferase (FT) using SPA assay |
Bioorg Med Chem Lett 9: 703-8 (1999)
BindingDB Entry DOI: 10.7270/Q2G73CWQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data