 Found 976 hits for UniProtKB: P62942
Found 976 hits for UniProtKB: P62942 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM23334

(3-(pyridin-3-yl)propyl (2S)-1-(3,3-dimethyl-2-oxop...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)OCCCc1cccnc1 |r| Show InChI InChI=1S/C20H28N2O4/c1-4-20(2,3)17(23)18(24)22-12-6-10-16(22)19(25)26-13-7-9-15-8-5-11-21-14-15/h5,8,11,14,16H,4,6-7,9-10,12-13H2,1-3H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36609
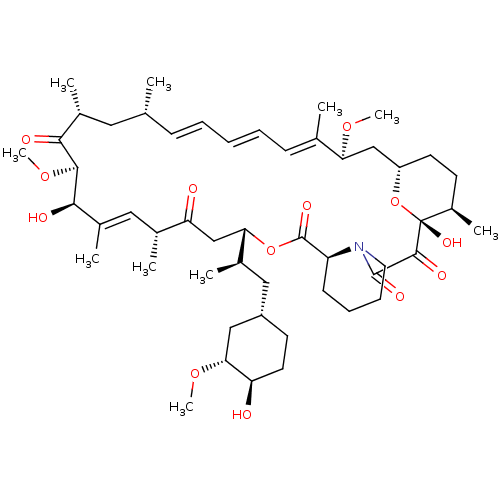
(Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50517882
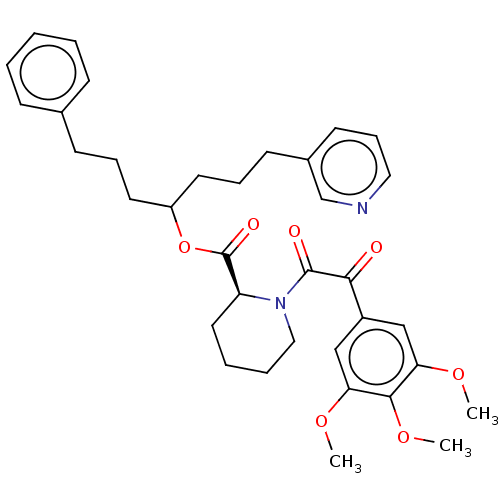
(CHEMBL4449096)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(CCCc1ccccc1)CCCc1cccnc1 |r| Show InChI InChI=1S/C35H42N2O7/c1-41-30-22-27(23-31(42-2)33(30)43-3)32(38)34(39)37-21-8-7-19-29(37)35(40)44-28(17-9-14-25-12-5-4-6-13-25)18-10-15-26-16-11-20-36-24-26/h4-6,11-13,16,20,22-24,28-29H,7-10,14-15,17-19,21H2,1-3H3/t28?,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of His6 tagged human FKBP12 expressed in Escherichia coli BL21(DE3) cells using succinylALPF-p-nitroanilide as substrate by fluorescence p... |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50030448
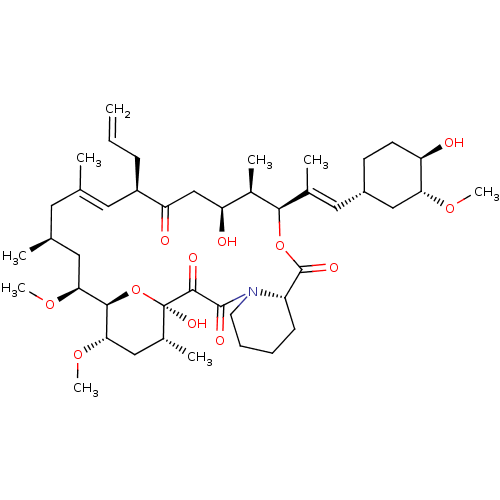
(8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...)Show SMILES CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC |r,t:45| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50263407
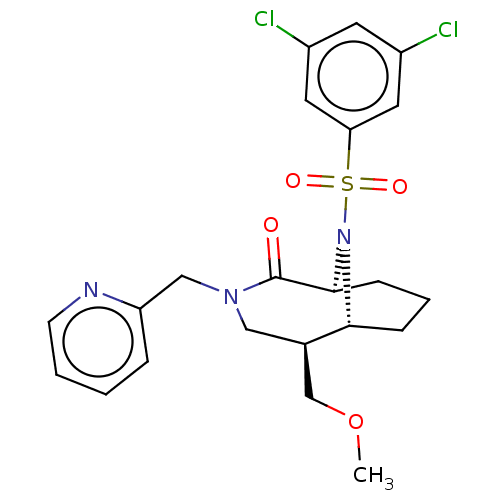
(CHEMBL4090599)Show SMILES [H][C@]12CCC[C@]([H])(N1S(=O)(=O)c1cc(Cl)cc(Cl)c1)C(=O)N(Cc1ccccn1)C[C@@H]2COC |r,TLB:31:30:7:2.3.4,20:19:7:2.3.4,THB:22:21:7:2.3.4| Show InChI InChI=1S/C22H25Cl2N3O4S/c1-31-14-15-12-26(13-18-5-2-3-8-25-18)22(28)21-7-4-6-20(15)27(21)32(29,30)19-10-16(23)9-17(24)11-19/h2-3,5,8-11,15,20-21H,4,6-7,12-14H2,1H3/t15-,20-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany.
Curated by ChEMBL
| Assay Description
Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... |
J Med Chem 61: 3660-3673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00137
BindingDB Entry DOI: 10.7270/Q2833VGW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068608
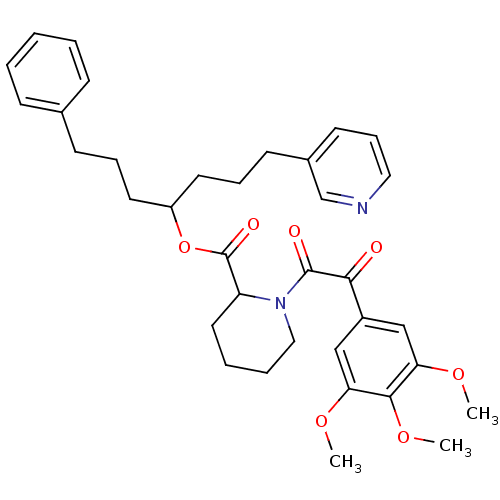
(1-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-acetyl]-piper...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCCCC1C(=O)OC(CCCc1ccccc1)CCCc1cccnc1 Show InChI InChI=1S/C35H42N2O7/c1-41-30-22-27(23-31(42-2)33(30)43-3)32(38)34(39)37-21-8-7-19-29(37)35(40)44-28(17-9-14-25-12-5-4-6-13-25)18-10-15-26-16-11-20-36-24-26/h4-6,11-13,16,20,22-24,28-29H,7-10,14-15,17-19,21H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36608

(Rapamycin C-7, analog 1)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42-,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408684
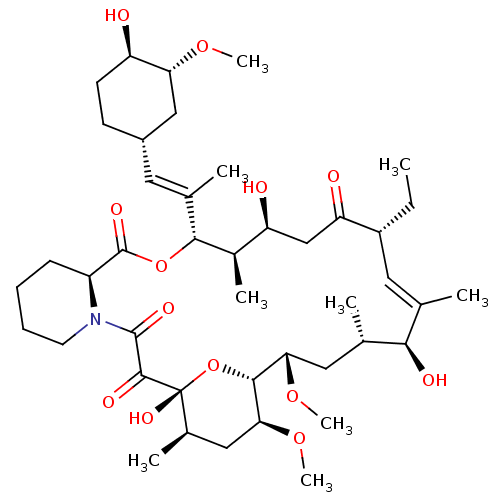
(CHEMBL2052020 | L-685818)Show SMILES CC[C@@H]1\C=C(C)/[C@@H](O)[C@@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OC |t:3| Show InChI InChI=1S/C43H69NO13/c1-10-29-18-23(2)37(48)24(3)19-35(54-8)39-36(55-9)20-26(5)43(52,57-39)40(49)41(50)44-16-12-11-13-30(44)42(51)56-38(27(6)32(46)22-33(29)47)25(4)17-28-14-15-31(45)34(21-28)53-7/h17-18,24,26-32,34-39,45-46,48,52H,10-16,19-22H2,1-9H3/b23-18-,25-17+/t24-,26+,27+,28-,29+,30-,31+,32-,34+,35-,36-,37+,38+,39+,43+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068597
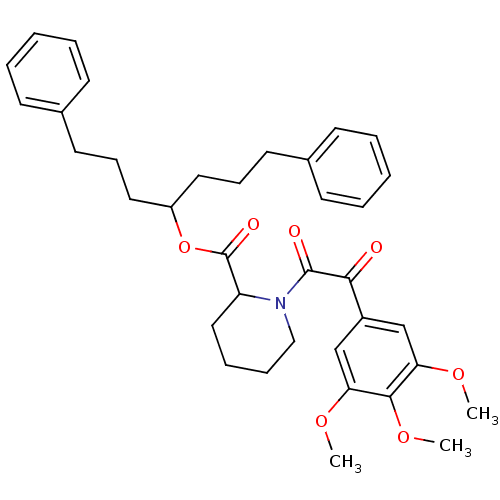
(1-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-acetyl]-piper...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCCCC1C(=O)OC(CCCc1ccccc1)CCCc1ccccc1 Show InChI InChI=1S/C36H43NO7/c1-41-31-24-28(25-32(42-2)34(31)43-3)33(38)35(39)37-23-11-10-22-30(37)36(40)44-29(20-12-18-26-14-6-4-7-15-26)21-13-19-27-16-8-5-9-17-27/h4-9,14-17,24-25,29-30H,10-13,18-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36627

(Rapamycin C-7, analog 16a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OCc2ccc(Cl)cc2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C57H82ClNO13/c1-34-15-11-10-12-16-35(2)48(70-33-41-19-22-43(58)23-20-41)31-44-24-18-40(7)57(67,72-44)54(64)55(65)59-26-14-13-17-45(59)56(66)71-49(37(4)29-42-21-25-46(60)50(30-42)68-8)32-47(61)36(3)28-39(6)52(63)53(69-9)51(62)38(5)27-34/h10-12,15-16,19-20,22-23,28,34,36-38,40,42,44-46,48-50,52-53,60,63,67H,13-14,17-18,21,24-27,29-33H2,1-9H3/b12-10+,15-11+,35-16+,39-28+/t34-,36-,37-,38-,40-,42+,44+,45+,46-,48-,49+,50-,52-,53+,57-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36612
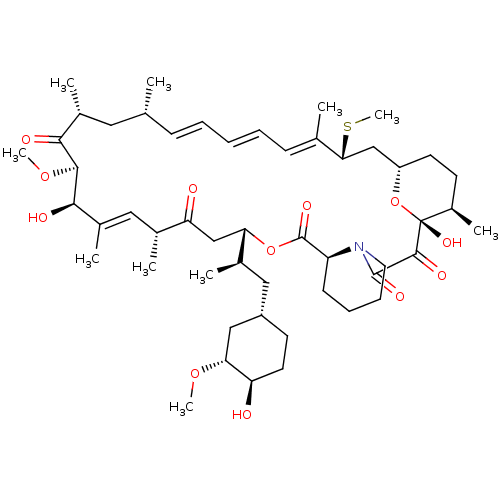
(Rapamycin C-7, analog 6a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)SC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO12S/c1-30-16-12-11-13-17-31(2)44(65-10)28-38-21-19-36(7)51(60,64-38)48(57)49(58)52-23-15-14-18-39(52)50(59)63-42(33(4)26-37-20-22-40(53)43(27-37)61-8)29-41(54)32(3)25-35(6)46(56)47(62-9)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43-,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36623
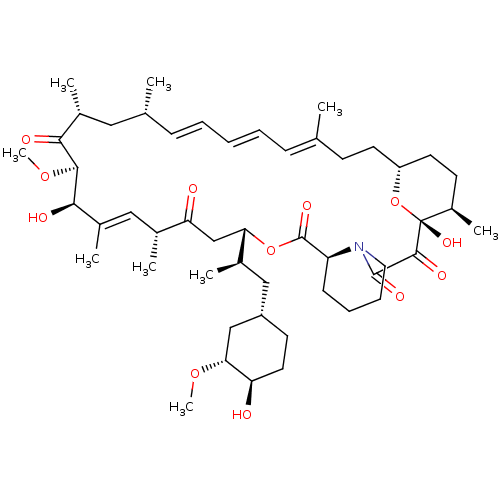
(Rapamycin C-7, analog 12)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\CC[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C50H77NO12/c1-30-15-11-10-12-16-31(2)25-34(5)44(54)46(61-9)45(55)35(6)26-32(3)41(53)29-42(33(4)27-37-20-23-40(52)43(28-37)60-8)62-49(58)39-17-13-14-24-51(39)48(57)47(56)50(59)36(7)19-22-38(63-50)21-18-30/h10-12,15-16,26,31-34,36-40,42-43,45-46,52,55,59H,13-14,17-25,27-29H2,1-9H3/b11-10+,16-12+,30-15+,35-26+/t31-,32-,33-,34-,36-,37+,38-,39+,40-,42+,43-,45-,46+,50-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068556
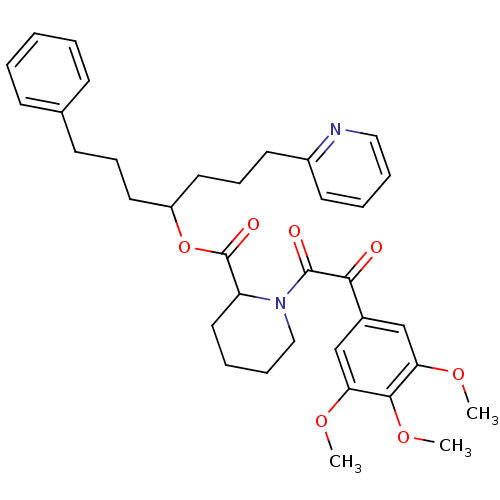
(1-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-acetyl]-piper...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCCCC1C(=O)OC(CCCc1ccccc1)CCCc1ccccn1 Show InChI InChI=1S/C35H42N2O7/c1-41-30-23-26(24-31(42-2)33(30)43-3)32(38)34(39)37-22-10-8-20-29(37)35(40)44-28(18-11-15-25-13-5-4-6-14-25)19-12-17-27-16-7-9-21-36-27/h4-7,9,13-14,16,21,23-24,28-29H,8,10-12,15,17-20,22H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113103
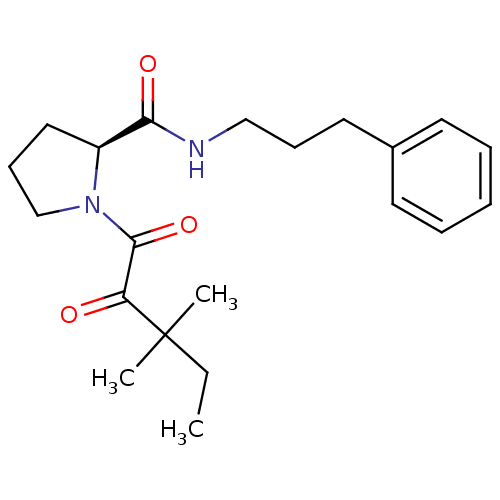
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)NCCCc1ccccc1 Show InChI InChI=1S/C21H30N2O3/c1-4-21(2,3)18(24)20(26)23-15-9-13-17(23)19(25)22-14-8-12-16-10-6-5-7-11-16/h5-7,10-11,17H,4,8-9,12-15H2,1-3H3,(H,22,25)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36613
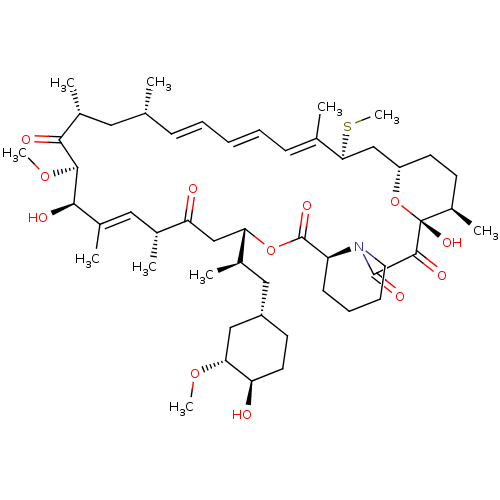
(Rapamycin C-7, analog 6b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)SC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO12S/c1-30-16-12-11-13-17-31(2)44(65-10)28-38-21-19-36(7)51(60,64-38)48(57)49(58)52-23-15-14-18-39(52)50(59)63-42(33(4)26-37-20-22-40(53)43(27-37)61-8)29-41(54)32(3)25-35(6)46(56)47(62-9)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43-,44+,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36609

(Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36622

(Rapamycin C-7, analog 11b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2ccc[nH]2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C54H80N2O12/c1-32-16-11-10-12-17-33(2)41(42-18-15-24-55-42)30-40-22-20-38(7)54(64,68-40)51(61)52(62)56-25-14-13-19-43(56)53(63)67-46(35(4)28-39-21-23-44(57)47(29-39)65-8)31-45(58)34(3)27-37(6)49(60)50(66-9)48(59)36(5)26-32/h10-12,15-18,24,27,32,34-36,38-41,43-44,46-47,49-50,55,57,60,64H,13-14,19-23,25-26,28-31H2,1-9H3/b12-10+,16-11+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43+,44-,46+,47-,49-,50+,54-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068564
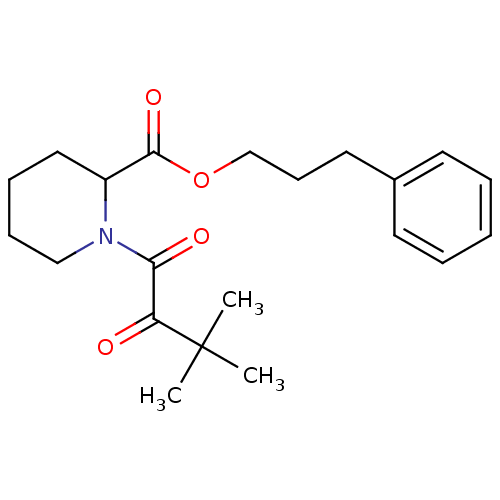
(1-(3,3-Dimethyl-2-oxo-butyryl)-piperidine-2-carbox...)Show SMILES CC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)OCCCc1ccccc1 Show InChI InChI=1S/C21H29NO4/c1-21(2,3)18(23)19(24)22-14-8-7-13-17(22)20(25)26-15-9-12-16-10-5-4-6-11-16/h4-6,10-11,17H,7-9,12-15H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
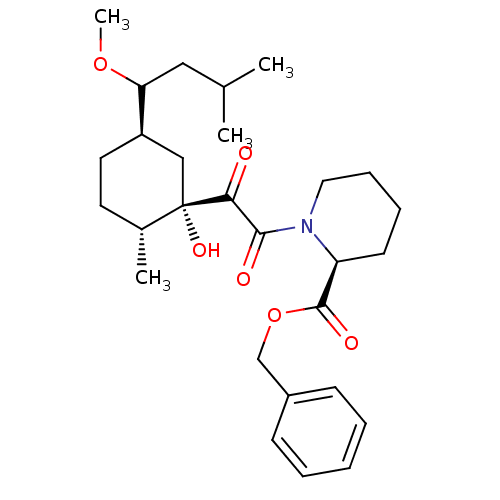
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403490
(CHEMBL312157)Show SMILES COC(CC(C)C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OCc1ccccc1 Show InChI InChI=1S/C28H41NO6/c1-19(2)16-24(34-4)22-14-13-20(3)28(33,17-22)25(30)26(31)29-15-9-8-12-23(29)27(32)35-18-21-10-6-5-7-11-21/h5-7,10-11,19-20,22-24,33H,8-9,12-18H2,1-4H3/t20-,22-,23+,24?,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against FK506 binding protein 12 was determined |
Bioorg Med Chem Lett 5: 2489-2494 (1995)
Article DOI: 10.1016/0960-894X(95)00429-W
BindingDB Entry DOI: 10.7270/Q24Q7W66 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068590

(1-(3,3-Dimethyl-2-oxo-butyryl)-pyrrolidine-2-carbo...)Show InChI InChI=1S/C19H26N2O4/c1-19(2,3)16(22)17(23)21-11-5-9-15(21)18(24)25-12-6-8-14-7-4-10-20-13-14/h4,7,10,13,15H,5-6,8-9,11-12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
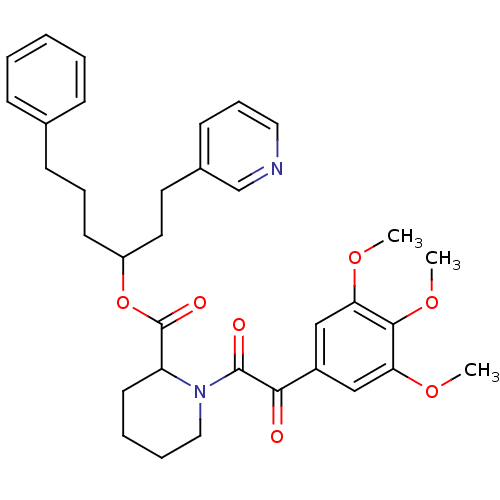
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068578
(1-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-acetyl]-piper...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCCCC1C(=O)OC(CCCc1ccccc1)CCc1cccnc1 Show InChI InChI=1S/C34H40N2O7/c1-40-29-21-26(22-30(41-2)32(29)42-3)31(37)33(38)36-20-8-7-16-28(36)34(39)43-27(18-17-25-14-10-19-35-23-25)15-9-13-24-11-5-4-6-12-24/h4-6,10-12,14,19,21-23,27-28H,7-9,13,15-18,20H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
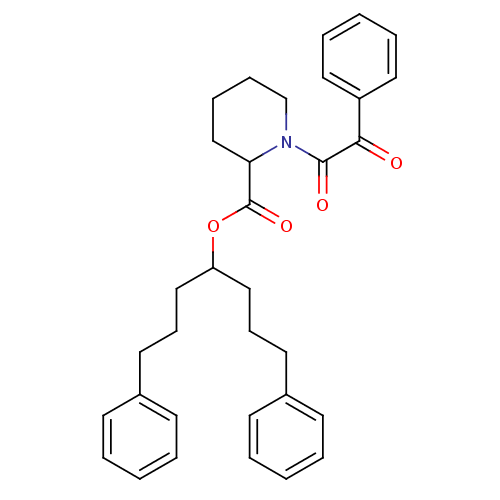
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068573
(1-(2-Oxo-2-phenyl-acetyl)-piperidine-2-carboxylic ...)Show SMILES O=C(OC(CCCc1ccccc1)CCCc1ccccc1)C1CCCCN1C(=O)C(=O)c1ccccc1 Show InChI InChI=1S/C33H37NO4/c35-31(28-20-8-3-9-21-28)32(36)34-25-11-10-24-30(34)33(37)38-29(22-12-18-26-14-4-1-5-15-26)23-13-19-27-16-6-2-7-17-27/h1-9,14-17,20-21,29-30H,10-13,18-19,22-25H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36619
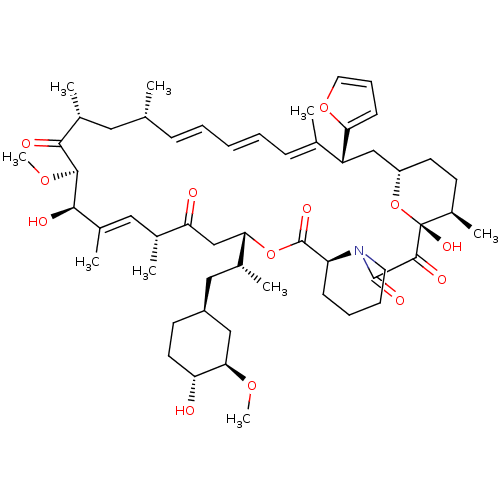
(Rapamycin C-7, analog 10a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2ccco2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C54H79NO13/c1-32-16-11-10-12-17-33(2)41(45-19-15-25-66-45)30-40-22-20-38(7)54(63,68-40)51(60)52(61)55-24-14-13-18-42(55)53(62)67-46(35(4)28-39-21-23-43(56)47(29-39)64-8)31-44(57)34(3)27-37(6)49(59)50(65-9)48(58)36(5)26-32/h10-12,15-17,19,25,27,32,34-36,38-43,46-47,49-50,56,59,63H,13-14,18,20-24,26,28-31H2,1-9H3/b12-10+,16-11+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41-,42+,43-,46+,47-,49-,50+,54-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36610
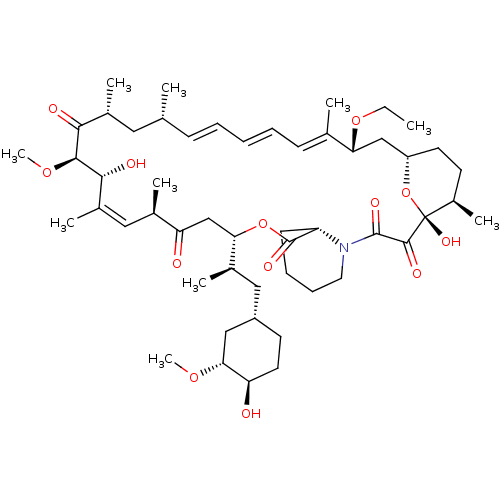
(Rapamycin C-7, analog 5a)Show SMILES CCO[C@@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H](CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:34,t:49,51,53| Show InChI InChI=1S/C52H81NO13/c1-11-64-43-29-39-22-20-37(8)52(61,66-39)49(58)50(59)53-24-16-15-19-40(53)51(60)65-44(34(5)27-38-21-23-41(54)45(28-38)62-9)30-42(55)33(4)26-36(7)47(57)48(63-10)46(56)35(6)25-31(2)17-13-12-14-18-32(43)3/h12-14,17-18,26,31,33-35,37-41,43-45,47-48,54,57,61H,11,15-16,19-25,27-30H2,1-10H3/b14-12+,17-13+,32-18+,36-26+/t31-,33-,34-,35-,37-,38+,39+,40+,41-,43-,44+,45-,47-,48+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36615
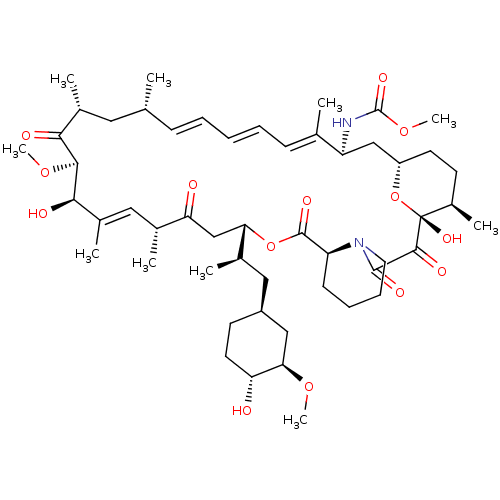
(Rapamycin C-7, analog 7b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)NC(=O)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C52H80N2O14/c1-30-16-12-11-13-17-31(2)39(53-51(62)66-10)28-38-21-19-36(7)52(63,68-38)48(59)49(60)54-23-15-14-18-40(54)50(61)67-43(33(4)26-37-20-22-41(55)44(27-37)64-8)29-42(56)32(3)25-35(6)46(58)47(65-9)45(57)34(5)24-30/h11-13,16-17,25,30,32-34,36-41,43-44,46-47,55,58,63H,14-15,18-24,26-29H2,1-10H3,(H,53,62)/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40+,41-,43+,44-,46-,47+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606717
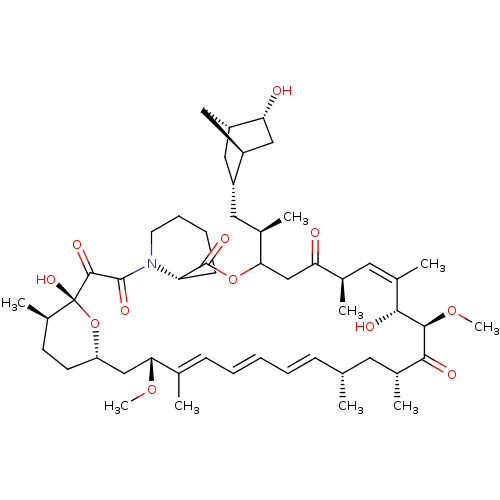
(CHEMBL5218737)Show SMILES [H][C@]12C[C@@H](C[C@@H](C)C3CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@]4([H])CC[C@@H](C)[C@@](O)(O4)C(=O)C(=O)N4CCCC[C@H]4C(=O)O3)OC)[C@]([H])(C[C@H]1O)C2 |r,c:13,32,t:28,30| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36611

(Rapamycin C-7, analog 5b)Show SMILES CCO[C@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H](CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:34,t:49,51,53| Show InChI InChI=1S/C52H81NO13/c1-11-64-43-29-39-22-20-37(8)52(61,66-39)49(58)50(59)53-24-16-15-19-40(53)51(60)65-44(34(5)27-38-21-23-41(54)45(28-38)62-9)30-42(55)33(4)26-36(7)47(57)48(63-10)46(56)35(6)25-31(2)17-13-12-14-18-32(43)3/h12-14,17-18,26,31,33-35,37-41,43-45,47-48,54,57,61H,11,15-16,19-25,27-30H2,1-10H3/b14-12+,17-13+,32-18+,36-26+/t31-,33-,34-,35-,37-,38+,39+,40+,41-,43+,44+,45-,47-,48+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 4 | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606714
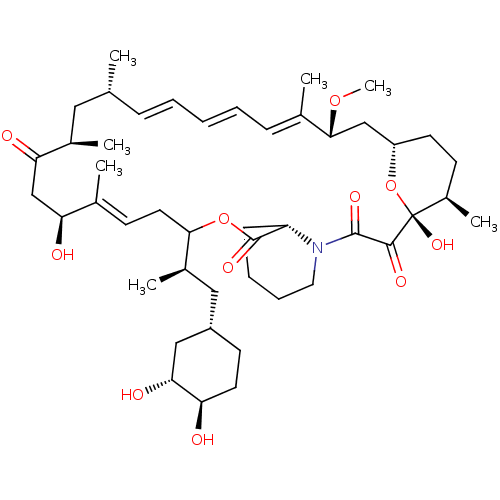
(CHEMBL5220794)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](O)[C@H](O)C1 |r,c:26,43,t:39,41| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257517
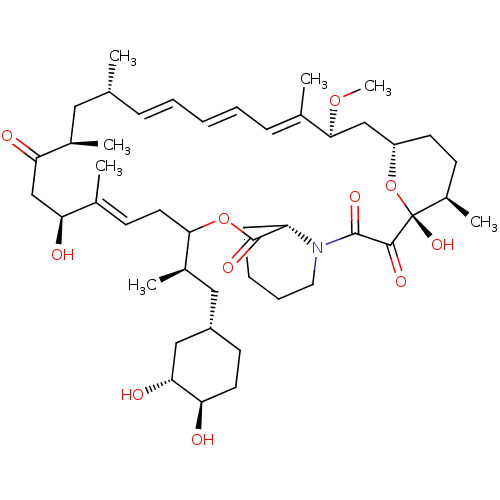
(US9505773, 10)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@@H]1CC[C@@H](O)[C@H](O)C1 |r,c:29,t:42,44,46| Show InChI InChI=1S/C46H71NO11/c1-28-13-9-8-10-14-30(3)42(56-7)26-35-19-17-33(6)46(55,58-35)43(52)44(53)47-22-12-11-15-36(47)45(54)57-41(32(5)24-34-18-20-37(48)40(51)25-34)21-16-29(2)38(49)27-39(50)31(4)23-28/h8-10,13-14,16,28,31-38,40-42,48-49,51,55H,11-12,15,17-27H2,1-7H3/b10-8+,13-9+,29-16+,30-14+/t28-,31-,32-,33-,34+,35+,36+,37-,38+,40-,41?,42+,46-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36614
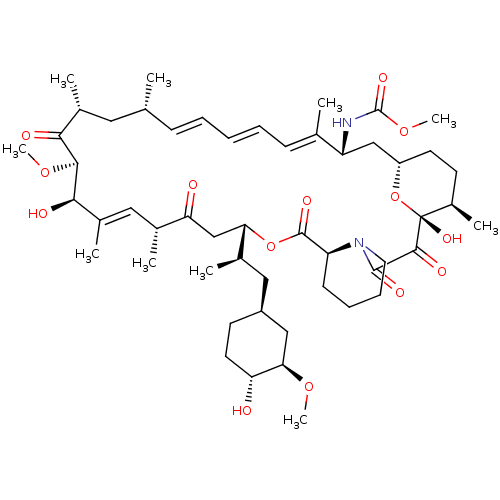
(Rapamycin C-7, analog 7a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)NC(=O)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C52H80N2O14/c1-30-16-12-11-13-17-31(2)39(53-51(62)66-10)28-38-21-19-36(7)52(63,68-38)48(59)49(60)54-23-15-14-18-40(54)50(61)67-43(33(4)26-37-20-22-41(55)44(27-37)64-8)29-42(56)32(3)25-35(6)46(58)47(65-9)45(57)34(5)24-30/h11-13,16-17,25,30,32-34,36-41,43-44,46-47,55,58,63H,14-15,18-24,26-29H2,1-10H3,(H,53,62)/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39-,40+,41-,43+,44-,46-,47+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068582
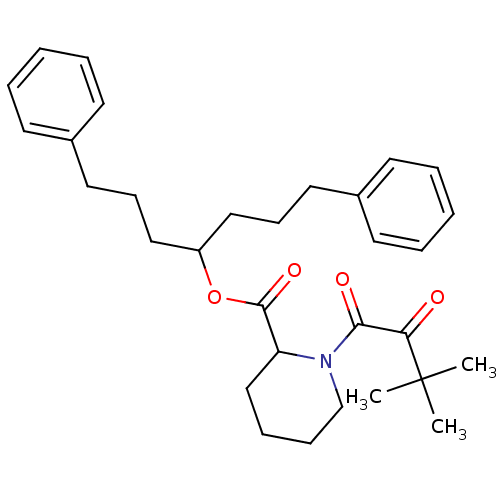
(1-(3,3-Dimethyl-2-oxo-butyryl)-piperidine-2-carbox...)Show SMILES CC(C)(C)C(=O)C(=O)N1CCCCC1C(=O)OC(CCCc1ccccc1)CCCc1ccccc1 Show InChI InChI=1S/C31H41NO4/c1-31(2,3)28(33)29(34)32-23-11-10-22-27(32)30(35)36-26(20-12-18-24-14-6-4-7-15-24)21-13-19-25-16-8-5-9-17-25/h4-9,14-17,26-27H,10-13,18-23H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit FK506 binding protein 12 rotamase activity |
J Med Chem 41: 5119-43 (1999)
Article DOI: 10.1021/jm980307x
BindingDB Entry DOI: 10.7270/Q22B8ZP8 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403336
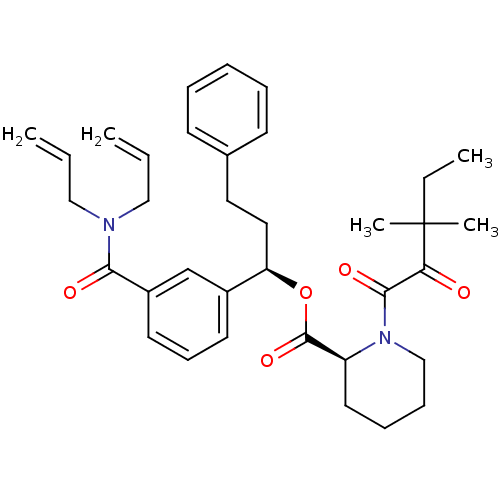
(CHEMBL326881)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)c1cccc(c1)C(=O)N(CC=C)CC=C Show InChI InChI=1S/C35H44N2O5/c1-6-22-36(23-7-2)32(39)28-18-14-17-27(25-28)30(21-20-26-15-10-9-11-16-26)42-34(41)29-19-12-13-24-37(29)33(40)31(38)35(4,5)8-3/h6-7,9-11,14-18,25,29-30H,1-2,8,12-13,19-24H2,3-5H3/t29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36625
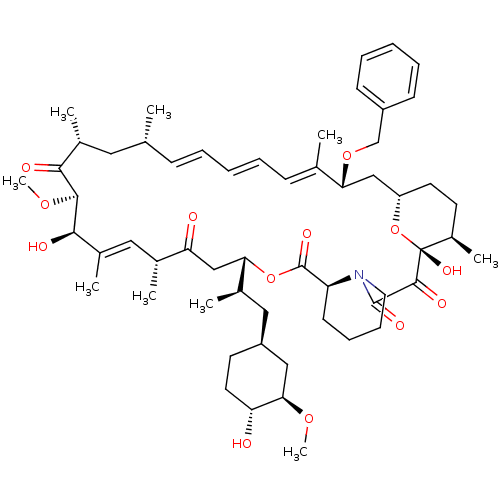
(Rapamycin C-7, analog 14a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OCc2ccccc2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C57H83NO13/c1-35-18-12-10-13-19-36(2)48(69-34-42-20-14-11-15-21-42)32-44-25-23-41(7)57(66,71-44)54(63)55(64)58-27-17-16-22-45(58)56(65)70-49(38(4)30-43-24-26-46(59)50(31-43)67-8)33-47(60)37(3)29-40(6)52(62)53(68-9)51(61)39(5)28-35/h10-15,18-21,29,35,37-39,41,43-46,48-50,52-53,59,62,66H,16-17,22-28,30-34H2,1-9H3/b13-10+,18-12+,36-19+,40-29+/t35-,37-,38-,39-,41-,43+,44+,45+,46-,48-,49+,50-,52-,53+,57-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403339
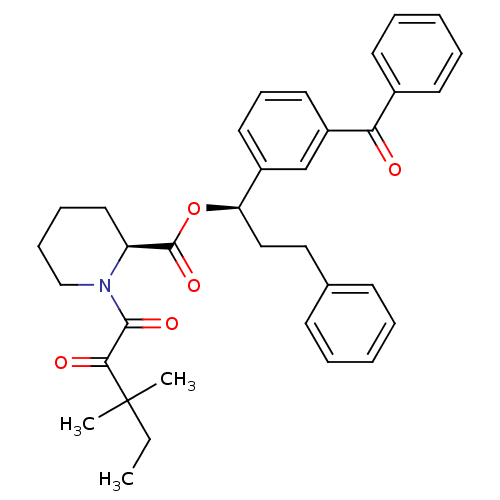
(CHEMBL109950)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)c1cccc(c1)C(=O)c1ccccc1 Show InChI InChI=1S/C35H39NO5/c1-4-35(2,3)32(38)33(39)36-23-12-11-20-29(36)34(40)41-30(22-21-25-14-7-5-8-15-25)27-18-13-19-28(24-27)31(37)26-16-9-6-10-17-26/h5-10,13-19,24,29-30H,4,11-12,20-23H2,1-3H3/t29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against FK506 binding protein 12 (FKBP12) |
Bioorg Med Chem Lett 4: 325-328 (1994)
Article DOI: 10.1016/S0960-894X(01)80137-2
BindingDB Entry DOI: 10.7270/Q21Z45M7 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM92863
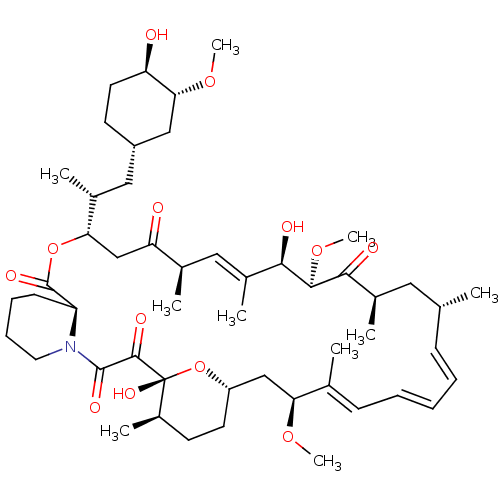
(US9505773, Rapamycin | mTOR Inhibitor, Rapamycin)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C/C=C/C=C(C)/[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,29,33,t:31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12-,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38?,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606713
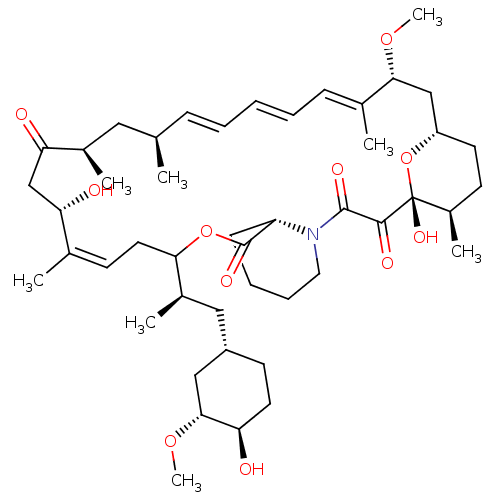
(CHEMBL5218786)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |r,c:26,43,t:39,41| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257515
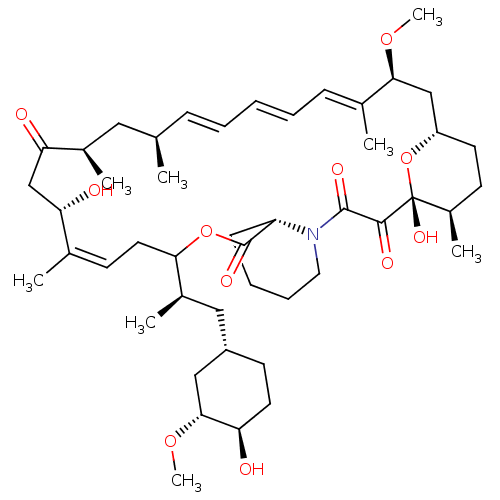
(US9505773, 8)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)C2C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |r,c:10,27,t:23,25| Show InChI InChI=1S/C47H73NO11/c1-29-14-10-9-11-15-31(3)42(56-7)27-36-20-18-34(6)47(55,59-36)44(52)45(53)48-23-13-12-16-37(48)46(54)58-41(22-17-30(2)39(50)28-40(51)32(4)24-29)33(5)25-35-19-21-38(49)43(26-35)57-8/h9-11,14-15,17,29,32-39,41-43,49-50,55H,12-13,16,18-28H2,1-8H3/b11-9+,14-10+,30-17+,31-15+/t29-,32-,33-,34-,35+,36+,37+,38-,39+,41?,42+,43-,47-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50116632
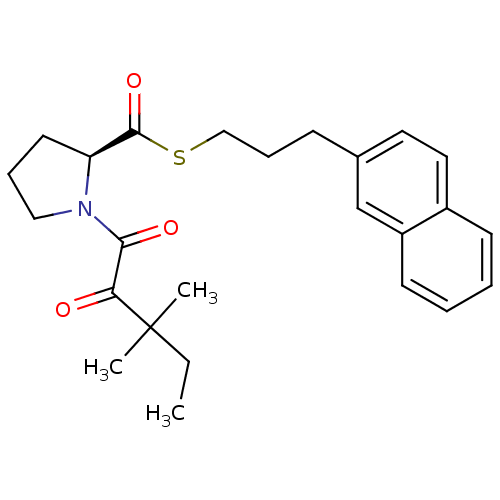
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)SCCCc1ccc2ccccc2c1 Show InChI InChI=1S/C25H31NO3S/c1-4-25(2,3)22(27)23(28)26-15-7-12-21(26)24(29)30-16-8-9-18-13-14-19-10-5-6-11-20(19)17-18/h5-6,10-11,13-14,17,21H,4,7-9,12,15-16H2,1-3H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against FK506 binding protein 12 |
J Med Chem 45: 3549-57 (2002)
BindingDB Entry DOI: 10.7270/Q2416WC5 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36620

(Rapamycin C-7, analog 10b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2ccco2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C54H79NO13/c1-32-16-11-10-12-17-33(2)41(45-19-15-25-66-45)30-40-22-20-38(7)54(63,68-40)51(60)52(61)55-24-14-13-18-42(55)53(62)67-46(35(4)28-39-21-23-43(56)47(29-39)64-8)31-44(57)34(3)27-37(6)49(59)50(65-9)48(58)36(5)26-32/h10-12,15-17,19,25,27,32,34-36,38-43,46-47,49-50,56,59,63H,13-14,18,20-24,26,28-31H2,1-9H3/b12-10+,16-11+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,42+,43-,46+,47-,49-,50+,54-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257521

(US9505773, 14)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H](CC(O)CC(=O)[C@H](C)\C=C(C)\[C@@H](O)CC(=O)[C@@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@H]1CC[C@H](O)CC1 |r,wU:21.22,7.7,9.9,37.39,42.44,60.63,wD:4.11,45.47,25.56,54.57,57.59,32.34,2.1,c:36,t:49,51,53,(-4.64,-8.27,;-3.3,-7.52,;-3.27,-5.98,;-4.67,-6.54,;-6,-5.78,;-7.34,-6.54,;-8.67,-5.78,;-8.67,-4.23,;-10,-3.47,;-7.34,-3.47,;-8.67,-2.69,;-6,-4.23,;-7.34,-1.93,;-8.67,-1.15,;-6,-1.15,;-4.67,-1.93,;-6,.38,;-7.34,1.15,;-7.34,2.69,;-6,3.47,;-4.67,2.69,;-4.67,1.15,;-3.33,.38,;-3.33,-1.15,;-2,1.15,;-2,2.69,;-.67,3.47,;.67,2.69,;.67,1.15,;2,3.47,;3.33,2.69,;3.33,1.15,;4.67,3.47,;4.67,5,;6,2.69,;6,1.15,;4.67,.38,;7.34,.38,;8.67,1.15,;7.34,-1.15,;8.67,-1.93,;10,-1.15,;8.67,-3.47,;7.34,-4.23,;7.34,-5.78,;6,-6.54,;6,-8.08,;4.67,-5.78,;3.33,-6.54,;2,-5.78,;.67,-6.54,;-.67,-5.78,;-2,-6.54,;-2,-8.08,;-3.33,3.47,;-4.67,4.23,;-3.33,5,;-2,5.78,;-.67,5,;.67,5.78,;.67,7.31,;2,8.08,;-.67,8.08,;-2,7.31,)| Show InChI InChI=1S/C51H79NO12/c1-31-14-10-9-11-15-32(2)46(62-8)29-41-22-17-37(7)51(61,64-41)48(58)49(59)52-23-13-12-16-42(52)50(60)63-47(36(6)26-38-18-20-39(53)21-19-38)28-40(54)27-43(55)34(4)25-35(5)45(57)30-44(56)33(3)24-31/h9-11,14-15,25,31,33-34,36-42,45-47,53-54,57,61H,12-13,16-24,26-30H2,1-8H3/b11-9+,14-10+,32-15+,35-25+/t31-,33+,34-,36-,37-,38-,39-,40?,41+,42+,45+,46+,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50606715
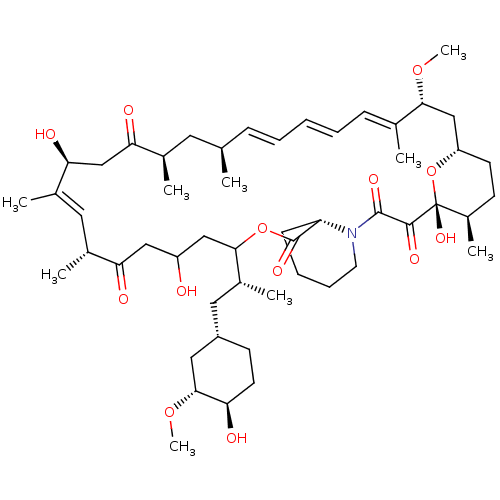
(CHEMBL5219513)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(CC(O)CC(=O)[C@H](C)\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |r,c:33,50,t:46,48| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50263416

(CHEMBL4067970)Show SMILES [H][C@]12CCC[C@]([H])(N1S(=O)(=O)c1cc(Cl)cc(Cl)c1)C(=O)N(CC(=O)OCC)C[C@@H]2C=C |r,TLB:20:19:7:2.3.4,30:29:7:2.3.4,THB:22:21:7:2.3.4| Show InChI InChI=1S/C20H24Cl2N2O5S/c1-3-13-11-23(12-19(25)29-4-2)20(26)18-7-5-6-17(13)24(18)30(27,28)16-9-14(21)8-15(22)10-16/h3,8-10,13,17-18H,1,4-7,11-12H2,2H3/t13-,17+,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany.
Curated by ChEMBL
| Assay Description
Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... |
J Med Chem 61: 3660-3673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00137
BindingDB Entry DOI: 10.7270/Q2833VGW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | CHEMBL5291300

| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50263435
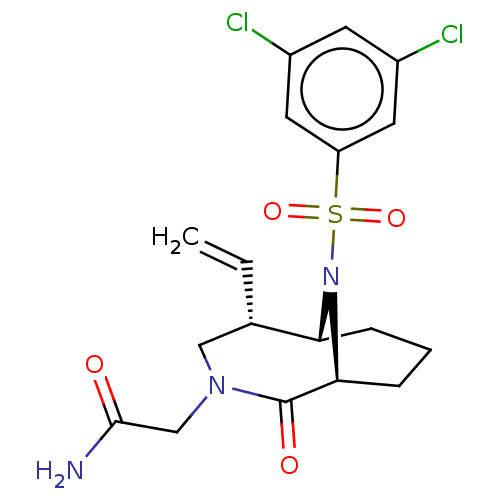
(CHEMBL4075704)Show SMILES [H][C@]12CCC[C@]([H])(N1S(=O)(=O)c1cc(Cl)cc(Cl)c1)C(=O)N(CC(N)=O)C[C@@H]2C=C |r,TLB:20:19:7:2.3.4,28:27:7:2.3.4,THB:22:21:7:2.3.4| Show InChI InChI=1S/C18H21Cl2N3O4S/c1-2-11-9-22(10-17(21)24)18(25)16-5-3-4-15(11)23(16)28(26,27)14-7-12(19)6-13(20)8-14/h2,6-8,11,15-16H,1,3-5,9-10H2,(H2,21,24)/t11-,15+,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany.
Curated by ChEMBL
| Assay Description
Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... |
J Med Chem 61: 3660-3673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00137
BindingDB Entry DOI: 10.7270/Q2833VGW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50263422

(CHEMBL4072643)Show SMILES [H][C@]12CCC[C@]([H])(N1S(=O)(=O)c1cc(Cl)cc(Cl)c1)C(=O)N(CC(O)=O)C[C@@H]2C=C |r,TLB:20:19:7:2.3.4,28:27:7:2.3.4,THB:22:21:7:2.3.4| Show InChI InChI=1S/C18H20Cl2N2O5S/c1-2-11-9-21(10-17(23)24)18(25)16-5-3-4-15(11)22(16)28(26,27)14-7-12(19)6-13(20)8-14/h2,6-8,11,15-16H,1,3-5,9-10H2,(H,23,24)/t11-,15+,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany.
Curated by ChEMBL
| Assay Description
Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... |
J Med Chem 61: 3660-3673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00137
BindingDB Entry DOI: 10.7270/Q2833VGW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50263421
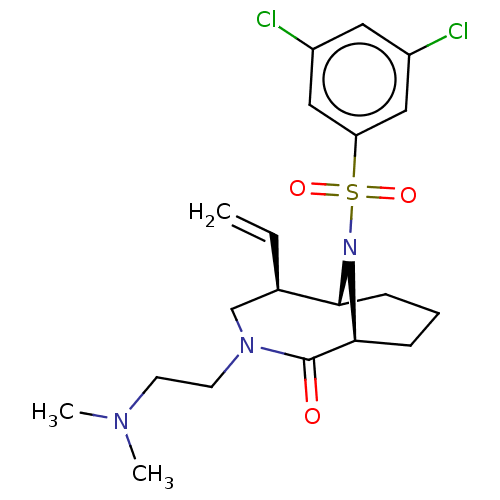
(CHEMBL4101268)Show SMILES [H][C@]12CCC[C@]([H])(N1S(=O)(=O)c1cc(Cl)cc(Cl)c1)C(=O)N(CCN(C)C)C[C@H]2C=C |r,TLB:20:19:7:2.3.4,29:28:7:2.3.4,THB:22:21:7:2.3.4| Show InChI InChI=1S/C20H27Cl2N3O3S/c1-4-14-13-24(9-8-23(2)3)20(26)19-7-5-6-18(14)25(19)29(27,28)17-11-15(21)10-16(22)12-17/h4,10-12,14,18-19H,1,5-9,13H2,2-3H3/t14-,18-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany.
Curated by ChEMBL
| Assay Description
Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... |
J Med Chem 61: 3660-3673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00137
BindingDB Entry DOI: 10.7270/Q2833VGW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50263420
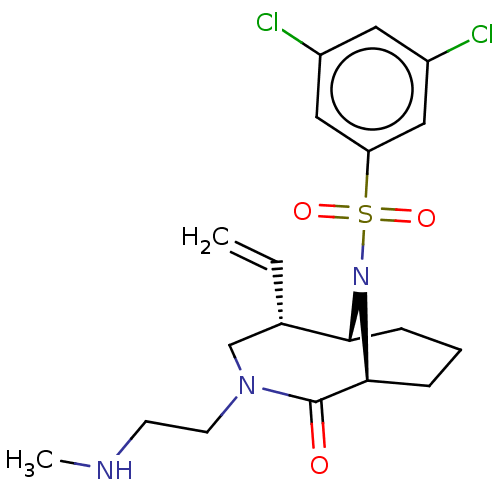
(CHEMBL4102121)Show SMILES [H][C@]12CCC[C@]([H])(N1S(=O)(=O)c1cc(Cl)cc(Cl)c1)C(=O)N(CCNC)C[C@@H]2C=C |r,TLB:20:19:7:2.3.4,28:27:7:2.3.4,THB:22:21:7:2.3.4| Show InChI InChI=1S/C19H25Cl2N3O3S/c1-3-13-12-23(8-7-22-2)19(25)18-6-4-5-17(13)24(18)28(26,27)16-10-14(20)9-15(21)11-16/h3,9-11,13,17-18,22H,1,4-8,12H2,2H3/t13-,17+,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany.
Curated by ChEMBL
| Assay Description
Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... |
J Med Chem 61: 3660-3673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00137
BindingDB Entry DOI: 10.7270/Q2833VGW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36626

(Rapamycin C-7, analog 15a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OCc2cccc(c2)N(=O)=O)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C57H82N2O15/c1-34-16-11-10-12-17-35(2)48(72-33-42-18-15-19-43(29-42)59(68)69)31-44-23-21-40(7)57(67,74-44)54(64)55(65)58-25-14-13-20-45(58)56(66)73-49(37(4)28-41-22-24-46(60)50(30-41)70-8)32-47(61)36(3)27-39(6)52(63)53(71-9)51(62)38(5)26-34/h10-12,15-19,27,29,34,36-38,40-41,44-46,48-50,52-53,60,63,67H,13-14,20-26,28,30-33H2,1-9H3/b12-10+,16-11+,35-17+,39-27+/t34-,36-,37-,38-,40-,41+,44+,45+,46-,48-,49+,50-,52-,53+,57-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36618

(Rapamycin C-7, analog 9)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2c(OC)cc(OC)cc2OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C59H87NO15/c1-34-18-14-13-15-19-35(2)44(52-50(71-10)31-43(69-8)32-51(52)72-11)30-42-23-21-40(7)59(68,75-42)56(65)57(66)60-25-17-16-20-45(60)58(67)74-48(37(4)28-41-22-24-46(61)49(29-41)70-9)33-47(62)36(3)27-39(6)54(64)55(73-12)53(63)38(5)26-34/h13-15,18-19,27,31-32,34,36-38,40-42,44-46,48-49,54-55,61,64,68H,16-17,20-26,28-30,33H2,1-12H3/b15-13+,18-14+,35-19+,39-27+/t34-,36-,37-,38-,40-,41+,42+,44+,45+,46-,48+,49-,54-,55+,59-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50116633

(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)SCCC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C27H33NO3S/c1-4-27(2,3)24(29)25(30)28-18-11-16-23(28)26(31)32-19-17-22(20-12-7-5-8-13-20)21-14-9-6-10-15-21/h5-10,12-15,22-23H,4,11,16-19H2,1-3H3/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against FK506 binding protein 12 |
J Med Chem 45: 3549-57 (2002)
BindingDB Entry DOI: 10.7270/Q2416WC5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data