 Found 339 hits of affinity data for UniProtKB/TrEMBL: P78540
Found 339 hits of affinity data for UniProtKB/TrEMBL: P78540 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50350311
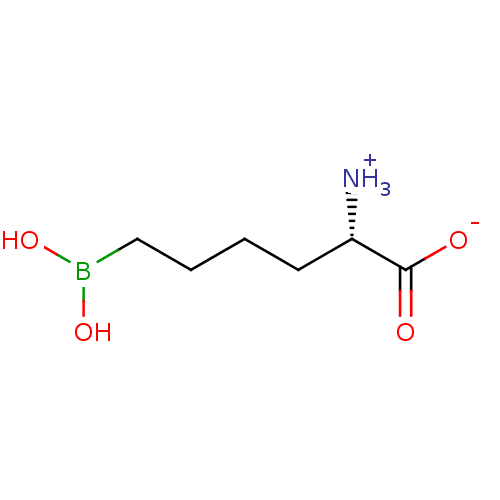
(CHEMBL1812661)Show InChI InChI=1S/C6H14BNO4/c8-5(6(9)10)3-1-2-4-7(11)12/h5,11-12H,1-4,8H2,(H,9,10)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University
Curated by ChEMBL
| Assay Description
Binding affinity to human arginase 2 |
J Med Chem 54: 5432-43 (2011)
Article DOI: 10.1021/jm200443b
BindingDB Entry DOI: 10.7270/Q2TH8N21 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50350311

(CHEMBL1812661)Show InChI InChI=1S/C6H14BNO4/c8-5(6(9)10)3-1-2-4-7(11)12/h5,11-12H,1-4,8H2,(H,9,10)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 at pH 7.5 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561046

(CHEMBL4790798) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 using L-arginine as substrate after 60 mins by spectrophotometric analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561045

(CHEMBL4758805)Show SMILES NC(CCCCB(O)O)(CCCN1CCC(O)(CC1)c1ccc(Cl)cc1)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 using L-arginine as substrate after 60 mins by spectrophotometric analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561034
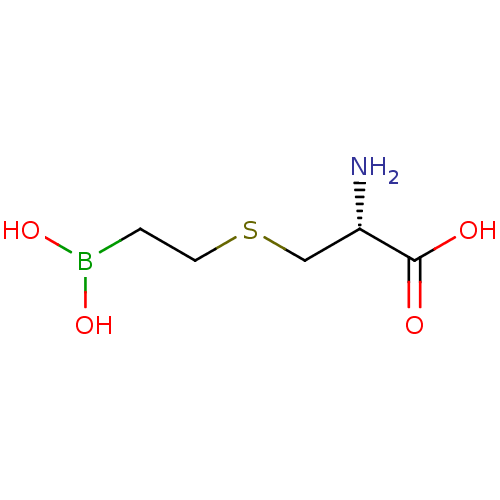
(CHEMBL4244287) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 at pH 7.5 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50008099
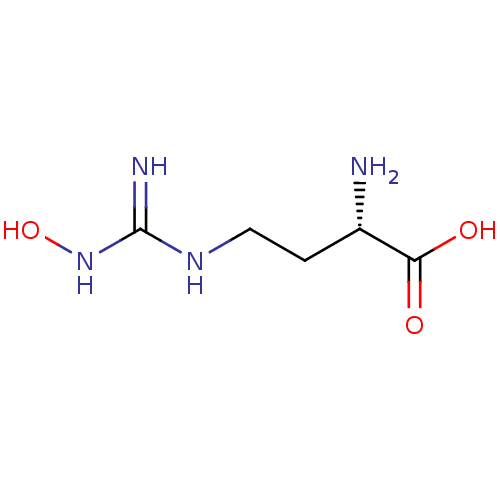
(CHEMBL1234777)Show InChI InChI=1S/C5H12N4O3/c6-3(4(10)11)1-2-8-5(7)9-12/h3,12H,1-2,6H2,(H,10,11)(H3,7,8,9)/t3-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 at pH 7.5 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50008099

(CHEMBL1234777)Show InChI InChI=1S/C5H12N4O3/c6-3(4(10)11)1-2-8-5(7)9-12/h3,12H,1-2,6H2,(H,10,11)(H3,7,8,9)/t3-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of arginase 2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2017.12.044
BindingDB Entry DOI: 10.7270/Q2736THR |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50294581
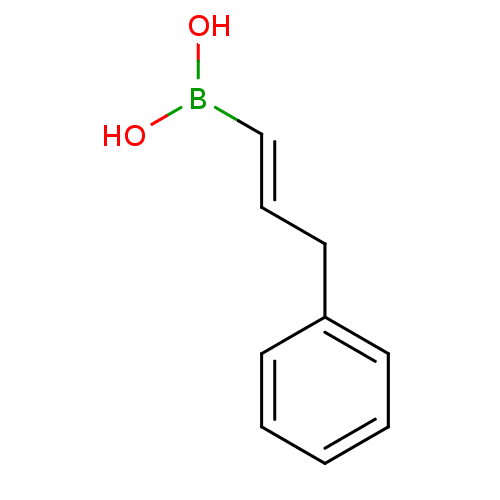
(3-phenylprop-1-enylboronic acid | CHEMBL539140)Show InChI InChI=1S/C9H11BO2/c11-10(12)8-4-7-9-5-2-1-3-6-9/h1-6,8,11-12H,7H2/b8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561034

(CHEMBL4244287) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50230418
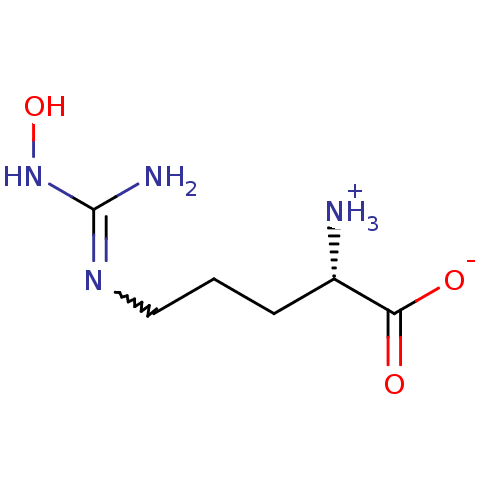
(CHEMBL260629 | N(gamma)-hydroxy-L-arginine | N-OME...)Show SMILES NC(NO)=NCCC[C@H]([NH3+])C([O-])=O |r,w:4.4| Show InChI InChI=1S/C6H14N4O3/c7-4(5(11)12)2-1-3-9-6(8)10-13/h4,13H,1-3,7H2,(H,11,12)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 at pH 7.5 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50462601
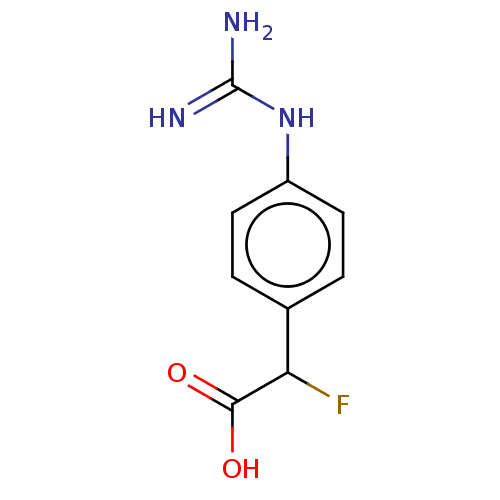
(CHEMBL4250607)Show InChI InChI=1S/C9H10FN3O2/c10-7(8(14)15)5-1-3-6(4-2-5)13-9(11)12/h1-4,7H,(H,14,15)(H4,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human arginase 2 using thioarginine as substrate measured up to 360 mins by UV micro plate method |
Bioorg Med Chem 26: 3939-3946 (2018)
Article DOI: 10.1016/j.bmc.2018.06.015
BindingDB Entry DOI: 10.7270/Q2TX3J1K |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50462600
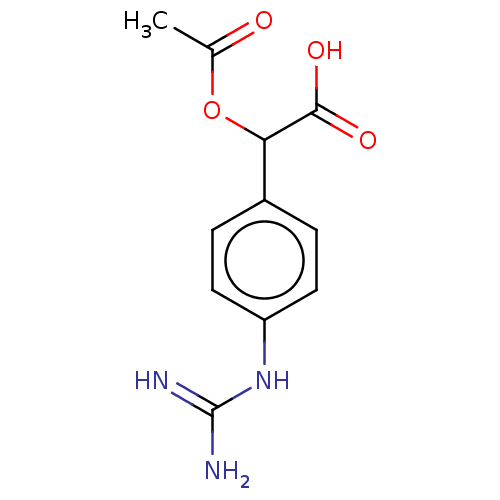
(CHEMBL4238387)Show InChI InChI=1S/C11H13N3O4/c1-6(15)18-9(10(16)17)7-2-4-8(5-3-7)14-11(12)13/h2-5,9H,1H3,(H,16,17)(H4,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human arginase 2 using thioarginine as substrate measured up to 360 mins by UV micro plate method |
Bioorg Med Chem 26: 3939-3946 (2018)
Article DOI: 10.1016/j.bmc.2018.06.015
BindingDB Entry DOI: 10.7270/Q2TX3J1K |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561047
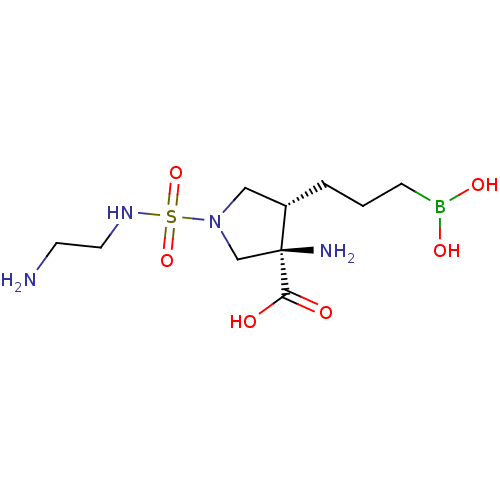
(CHEMBL4764455)Show SMILES NCCNS(=O)(=O)N1C[C@H](CCCB(O)O)[C@@](N)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642313
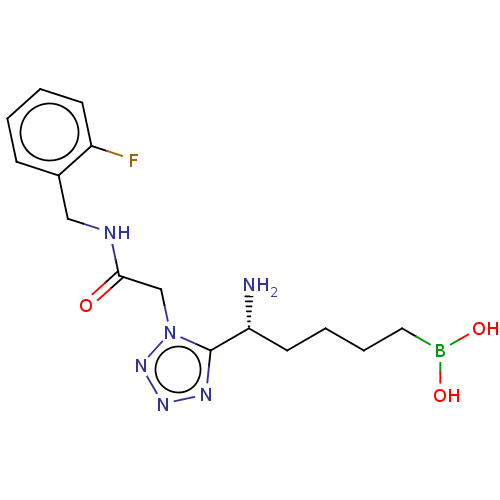
((+)-(R)-(5-amino-5-(1-(2-((2- fluorobenzyl)amino)-...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50547931

(CHEMBL4745275) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant arginase 2 expressed in Escherichia coli using thioarginine by Ellman's assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00593
BindingDB Entry DOI: 10.7270/Q2J106R8 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642315
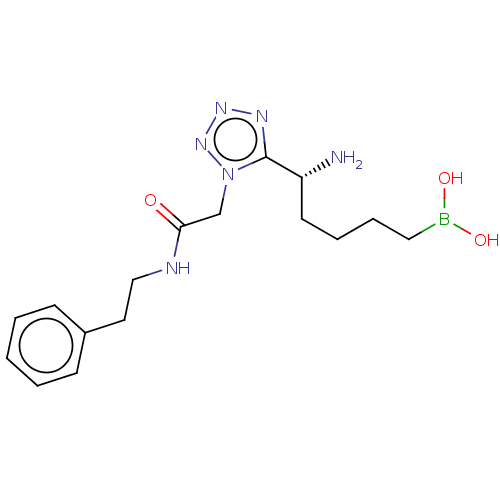
((+)-(R)-(5-amino-5-(1-(2-oxo-2- (phenethylamino)et...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642314
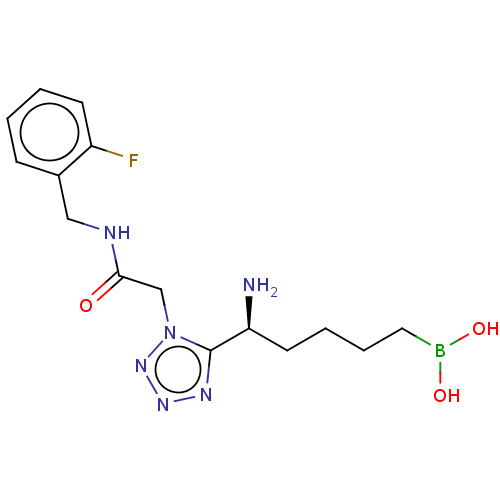
((-)-(S)-(5-amino-5-(1-(2-((2- fluorobenzyl)amino)-...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642312
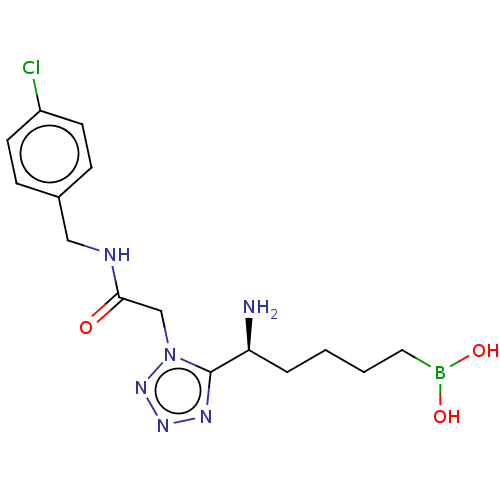
((-)-(S)-(5-amino-5-(1-(2-((4- chlorobenzyl)amino)-...)Show SMILES N[C@@H](CCCCB(O)O)c1nnnn1CC(=O)NCc1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642218
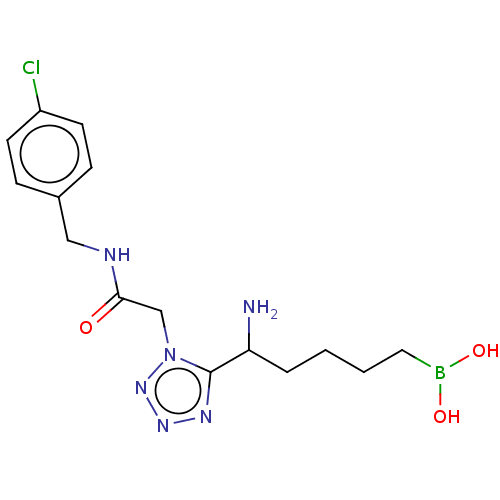
((5-amino-5-(1-(2-((4-chlorobenzyl) amino)-2-oxoeth...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642208
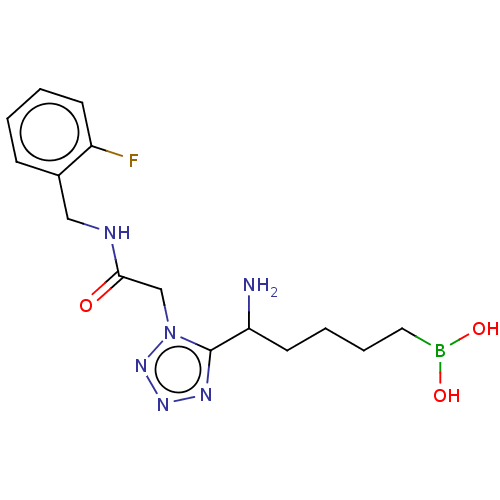
((5-amino-5-(1-(2-((2-fluorobenzyl)amino)- 2-oxoeth...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50350311

(CHEMBL1812661)Show InChI InChI=1S/C6H14BNO4/c8-5(6(9)10)3-1-2-4-7(11)12/h5,11-12H,1-4,8H2,(H,9,10)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561039

(CHEMBL4749355 | US11420984, Example 23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 by TOGA assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM568474
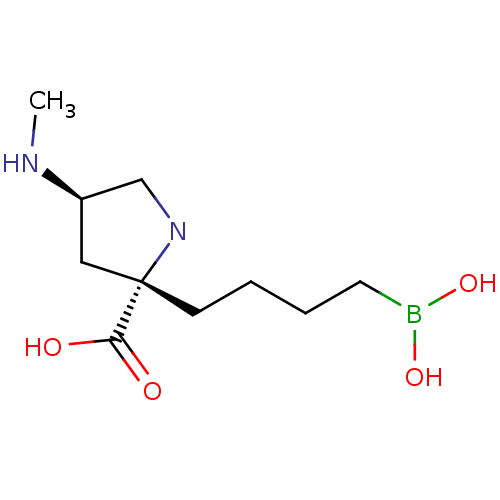
(US11420984, Example 24)Show SMILES CN[C@H]1CN[C@@](CCCCB(O)O)(C1)C(O)=O |r,$;;;;N;;;;;;;;;;;;$| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory effects of Examples 1 to 30 on the activity of Human Arginase 1 and Arginase 2 activity were quantified by measuring the formation of ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q243GV |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642316
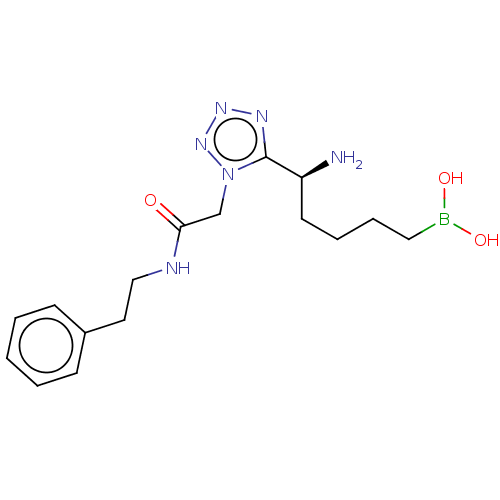
((-)-(S)-(5-amino-5-(1-(2-oxo-2- (phenethylamino)et...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642227
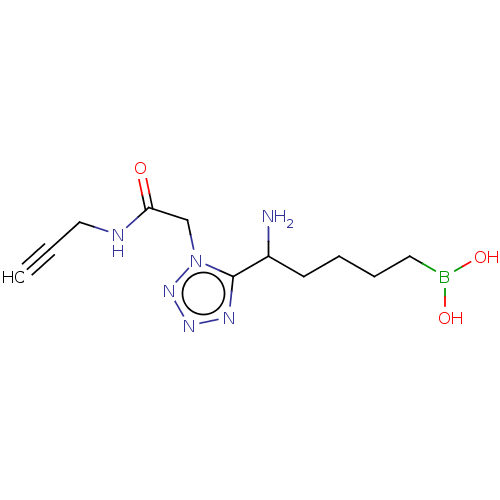
((5-amino-5-(1-(2-oxo-2-(prop-2-yn-1- ylamino)ethyl...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642221
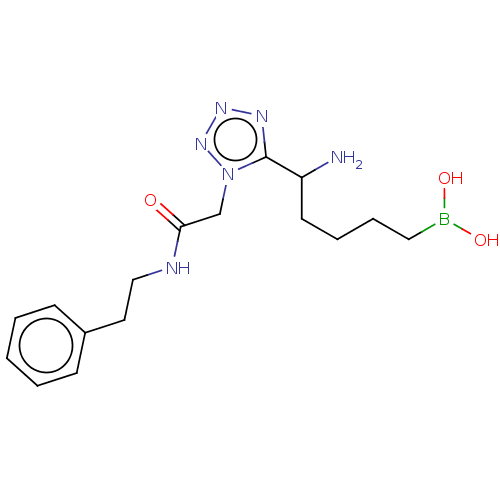
((5-Amino-5-(1-(2-oxo-2- (phenethylamino)ethyl)-1H-...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50547934
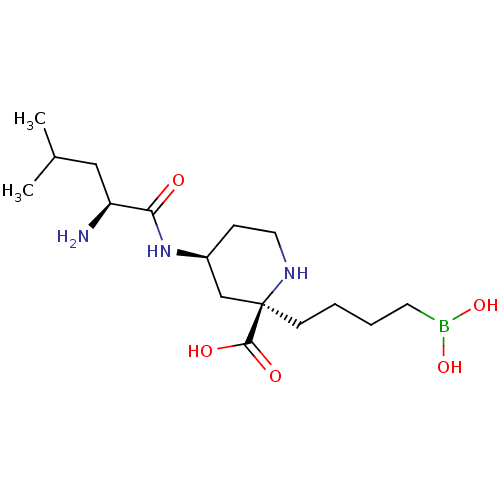
(CHEMBL4757930)Show SMILES CC(C)C[C@H](N)C(=O)N[C@H]1CCN[C@](CCCCB(O)O)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant arginase 2 expressed in Escherichia coli using thioarginine by Ellman's assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00593
BindingDB Entry DOI: 10.7270/Q2J106R8 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642273
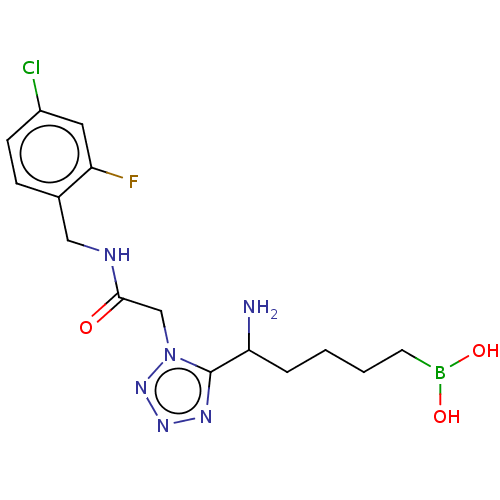
((5-amino-5-(1-(2-((4-chloro-2- fluorobenzyl)amino)...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561038
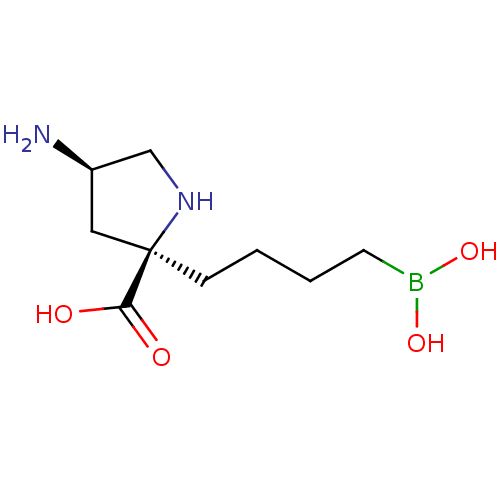
(CHEMBL4752307 | US11420984, Example 7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory effects of Examples 1 to 30 on the activity of Human Arginase 1 and Arginase 2 activity were quantified by measuring the formation of ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q243GV |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561038

(CHEMBL4752307 | US11420984, Example 7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 by TOGA assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50547935
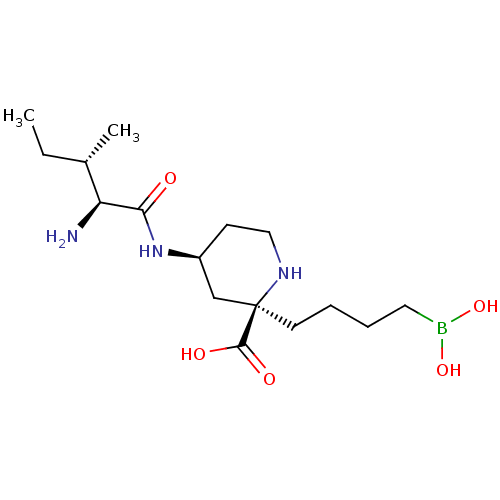
(CHEMBL4753285)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@H]1CCN[C@](CCCCB(O)O)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant arginase 2 expressed in Escherichia coli using thioarginine by Ellman's assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00593
BindingDB Entry DOI: 10.7270/Q2J106R8 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50547933
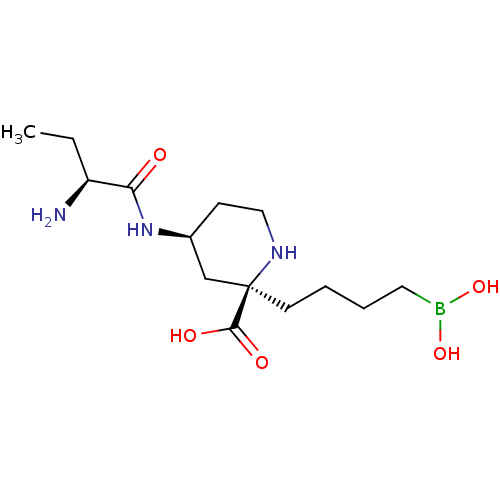
(CHEMBL4752391)Show SMILES CC[C@H](N)C(=O)N[C@H]1CCN[C@](CCCCB(O)O)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant arginase 2 expressed in Escherichia coli using thioarginine by Ellman's assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00593
BindingDB Entry DOI: 10.7270/Q2J106R8 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50439247
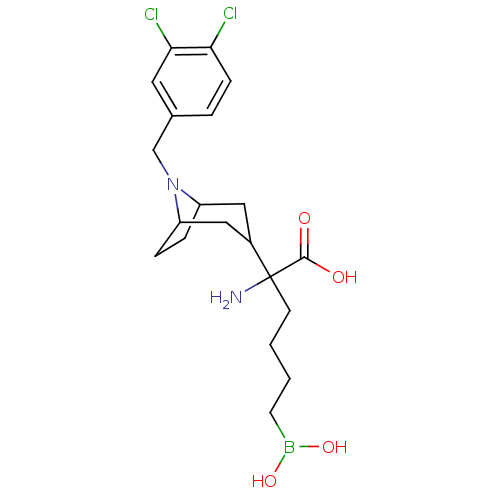
(CHEMBL2418831)Show SMILES NC(CCCCB(O)O)(C1CC2CCC(C1)N2Cc1ccc(Cl)c(Cl)c1)C(O)=O |TLB:17:16:12.13:9.10.15| Show InChI InChI=1S/C20H29BCl2N2O4/c22-17-6-3-13(9-18(17)23)12-25-15-4-5-16(25)11-14(10-15)20(24,19(26)27)7-1-2-8-21(28)29/h3,6,9,14-16,28-29H,1-2,4-5,7-8,10-12,24H2,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay |
Bioorg Med Chem Lett 23: 4837-41 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.092
BindingDB Entry DOI: 10.7270/Q2Z89DT6 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50547936

(CHEMBL4750174)Show SMILES CC(C)(C)[C@H](N)C(=O)N[C@H]1CCN[C@](CCCCB(O)O)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant arginase 2 expressed in Escherichia coli using thioarginine by Ellman's assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00593
BindingDB Entry DOI: 10.7270/Q2J106R8 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50547932
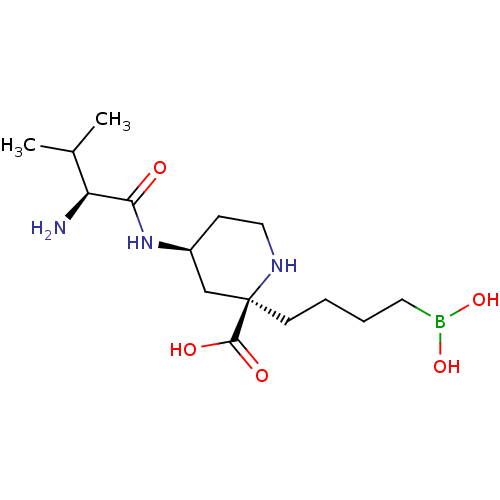
(CHEMBL4778086)Show SMILES CC(C)[C@H](N)C(=O)N[C@H]1CCN[C@](CCCCB(O)O)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant arginase 2 expressed in Escherichia coli using thioarginine by Ellman's assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00593
BindingDB Entry DOI: 10.7270/Q2J106R8 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642309
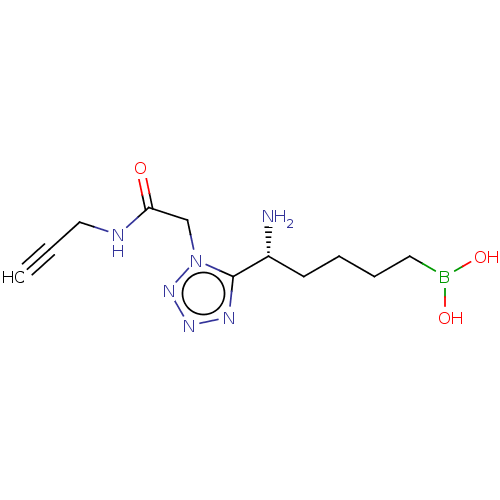
((+)-(R)-(5-amino-5-(1-(2-oxo-2-(prop-2- yn-1-ylami...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561034

(CHEMBL4244287) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50439246

(CHEMBL2418830)Show SMILES NC(CCCCB(O)O)(C1CC2CCC(C1)N2Cc1ccc(Cl)cc1)C(O)=O |TLB:17:16:12.13:9.10.15| Show InChI InChI=1S/C20H30BClN2O4/c22-16-5-3-14(4-6-16)13-24-17-7-8-18(24)12-15(11-17)20(23,19(25)26)9-1-2-10-21(27)28/h3-6,15,17-18,27-28H,1-2,7-13,23H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay |
Bioorg Med Chem Lett 23: 4837-41 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.092
BindingDB Entry DOI: 10.7270/Q2Z89DT6 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50439245
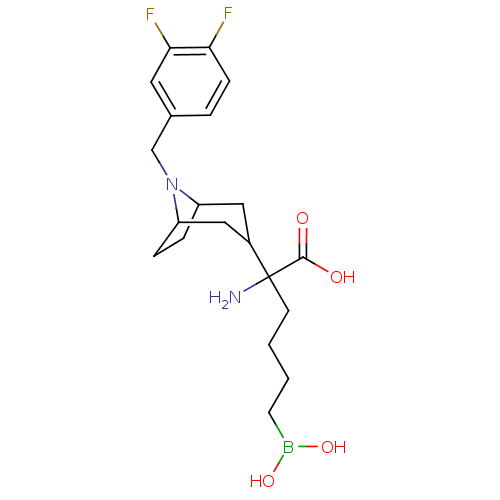
(CHEMBL2418991)Show SMILES NC(CCCCB(O)O)(C1CC2CCC(C1)N2Cc1ccc(F)c(F)c1)C(O)=O |TLB:17:16:12.13:9.10.15| Show InChI InChI=1S/C20H29BF2N2O4/c22-17-6-3-13(9-18(17)23)12-25-15-4-5-16(25)11-14(10-15)20(24,19(26)27)7-1-2-8-21(28)29/h3,6,9,14-16,28-29H,1-2,4-5,7-8,10-12,24H2,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay |
Bioorg Med Chem Lett 23: 4837-41 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.092
BindingDB Entry DOI: 10.7270/Q2Z89DT6 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642310
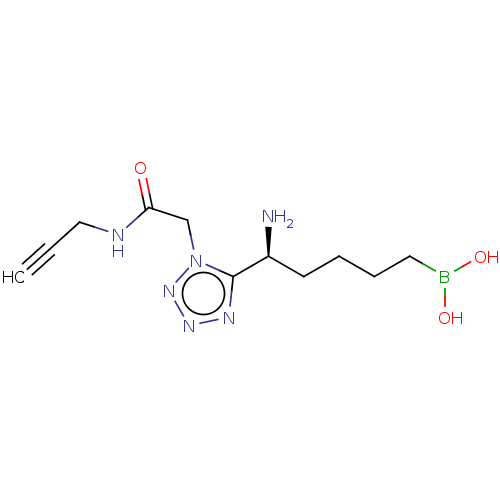
((-)-(S)-(5-amino-5-(1-(2-oxo-2-(prop-2- yn-1-ylami...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50439244
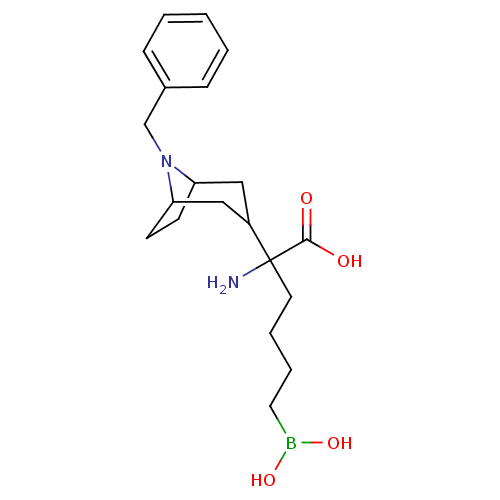
(CHEMBL2418829)Show SMILES NC(CCCCB(O)O)(C1CC2CCC(C1)N2Cc1ccccc1)C(O)=O |TLB:17:16:12.13:9.10.15| Show InChI InChI=1S/C20H31BN2O4/c22-20(19(24)25,10-4-5-11-21(26)27)16-12-17-8-9-18(13-16)23(17)14-15-6-2-1-3-7-15/h1-3,6-7,16-18,26-27H,4-5,8-14,22H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay |
Bioorg Med Chem Lett 23: 4837-41 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.092
BindingDB Entry DOI: 10.7270/Q2Z89DT6 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50439243
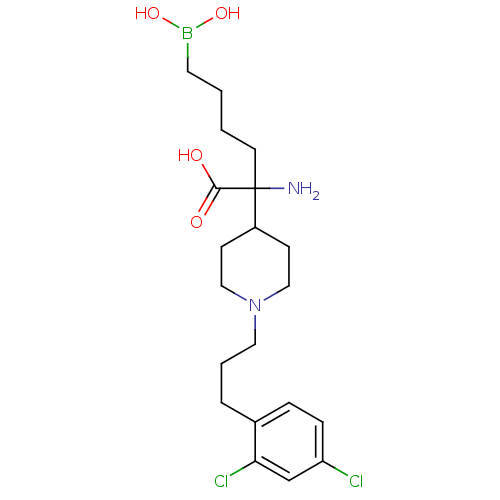
(CHEMBL2418998)Show SMILES NC(CCCCB(O)O)(C1CCN(CCCc2ccc(Cl)cc2Cl)CC1)C(O)=O Show InChI InChI=1S/C20H31BCl2N2O4/c22-17-6-5-15(18(23)14-17)4-3-11-25-12-7-16(8-13-25)20(24,19(26)27)9-1-2-10-21(28)29/h5-6,14,16,28-29H,1-4,7-13,24H2,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay |
Bioorg Med Chem Lett 23: 4837-41 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.092
BindingDB Entry DOI: 10.7270/Q2Z89DT6 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50008099

(CHEMBL1234777)Show InChI InChI=1S/C5H12N4O3/c6-3(4(10)11)1-2-8-5(7)9-12/h3,12H,1-2,6H2,(H,10,11)(H3,7,8,9)/t3-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642289
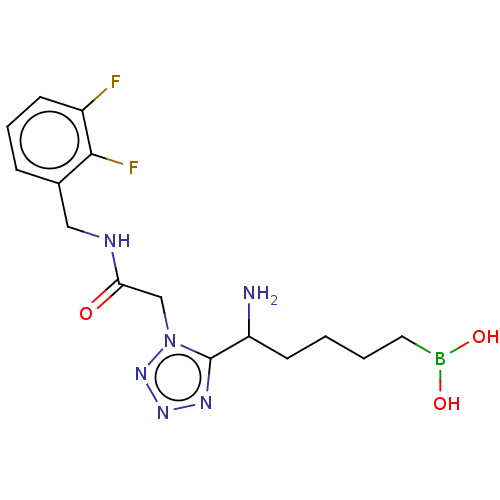
((5-amino-5-(1-(2-((2,3- difluorobenzyl)amino)-2-ox...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50427899
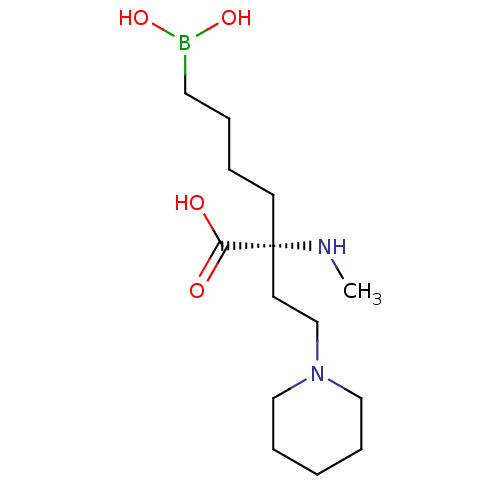
(CHEMBL2326090)Show InChI InChI=1S/C14H29BN2O4/c1-16-14(13(18)19,7-3-4-9-15(20)21)8-12-17-10-5-2-6-11-17/h16,20-21H,2-12H2,1H3,(H,18,19)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant fully active truncated form of arginase 2 overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea ... |
J Med Chem 56: 2568-80 (2013)
Article DOI: 10.1021/jm400014c
BindingDB Entry DOI: 10.7270/Q2BC40WP |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50439241

(CHEMBL2418999)Show SMILES NC(CCCCB(O)O)(C1CCN(CCCc2ccc(cc2)C(F)(F)F)CC1)C(O)=O Show InChI InChI=1S/C21H32BF3N2O4/c23-21(24,25)18-7-5-16(6-8-18)4-3-13-27-14-9-17(10-15-27)20(26,19(28)29)11-1-2-12-22(30)31/h5-8,17,30-31H,1-4,9-15,26H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay |
Bioorg Med Chem Lett 23: 4837-41 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.092
BindingDB Entry DOI: 10.7270/Q2Z89DT6 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50439242
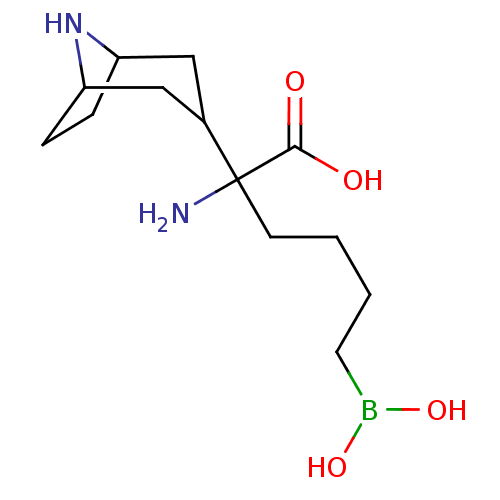
(CHEMBL2418828)Show InChI InChI=1S/C13H25BN2O4/c15-13(12(17)18,5-1-2-6-14(19)20)9-7-10-3-4-11(8-9)16-10/h9-11,16,19-20H,1-8,15H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay |
Bioorg Med Chem Lett 23: 4837-41 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.092
BindingDB Entry DOI: 10.7270/Q2Z89DT6 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50595730

(CHEMBL5171566)Show SMILES CC(C)C[C@H](N)C(=O)NC[C@@H]1CC[C@@H](CCB(O)O)C[C@]1(N)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00395
BindingDB Entry DOI: 10.7270/Q2VD73H4 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561049
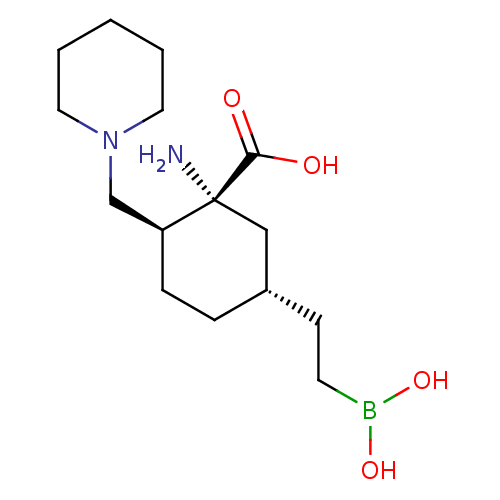
(CHEMBL4793482)Show SMILES N[C@@]1(C[C@H](CCB(O)O)CC[C@H]1CN1CCCCC1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM290388
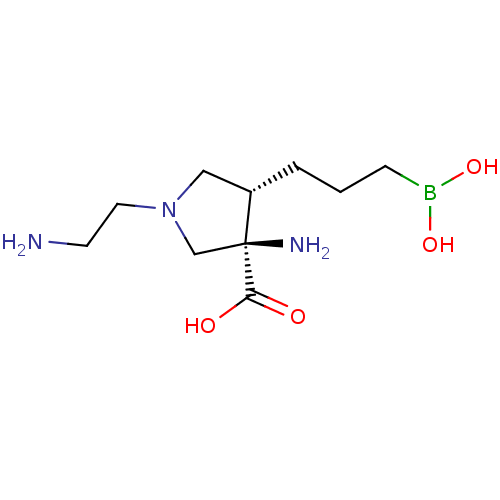
((3R,4S)-3-amino-1-(2-aminoethyl)-4-(3-boronopropyl...)Show SMILES NCCN1C[C@H](CCCB(O)O)[C@@](N)(C1)C(O)=O |r| Show InChI InChI=1S/C10H22BN3O4/c12-4-5-14-6-8(2-1-3-11(17)18)10(13,7-14)9(15)16/h8,17-18H,1-7,12-13H2,(H,15,16)/t8-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q289193N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data