

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50126960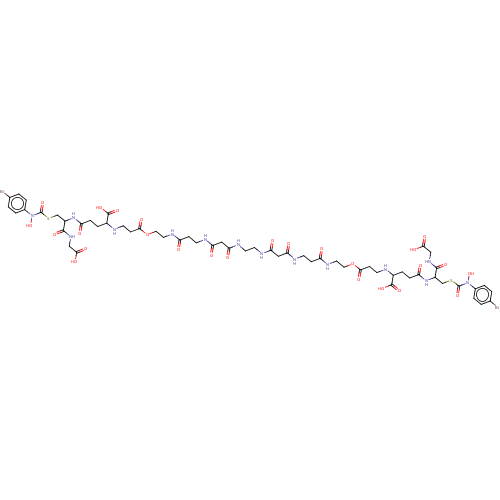 (CHEMBL3629116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of 6-His tagged recombinant human glyoxalase 1 transfected in Escherichia coli BL21 (DE3) assessed as S-D-lactoylglutathione formation by ... | Bioorg Med Chem Lett 25: 4724-7 (2015) Article DOI: 10.1016/j.bmcl.2015.08.055 BindingDB Entry DOI: 10.7270/Q26H4K7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50526945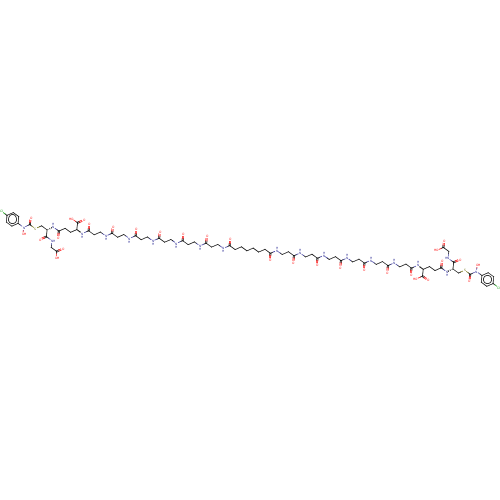 (CHEMBL4473806) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Michaelis-Menten analysis | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50126961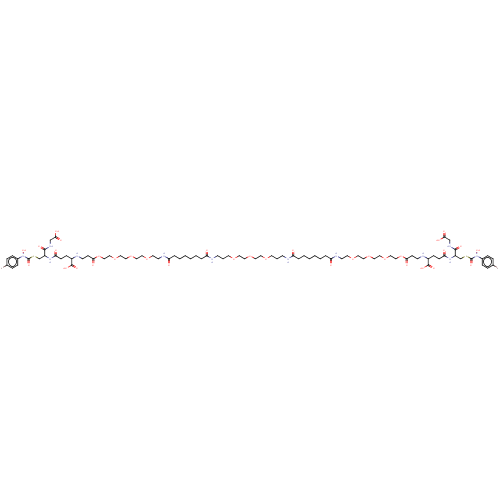 (CHEMBL3629115) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of 6-His tagged recombinant human glyoxalase 1 transfected in Escherichia coli BL21 (DE3) assessed as S-D-lactoylglutathione formation by ... | Bioorg Med Chem Lett 25: 4724-7 (2015) Article DOI: 10.1016/j.bmcl.2015.08.055 BindingDB Entry DOI: 10.7270/Q26H4K7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50526943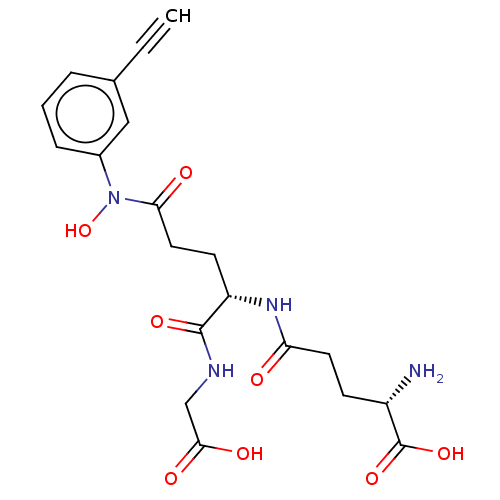 (CHEMBL4436073) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM16452 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Dixon plot analysis | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50140172 (CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte Glyoxalase-1 using GSH and MGO as substrate by Dixon plot analysis | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50526944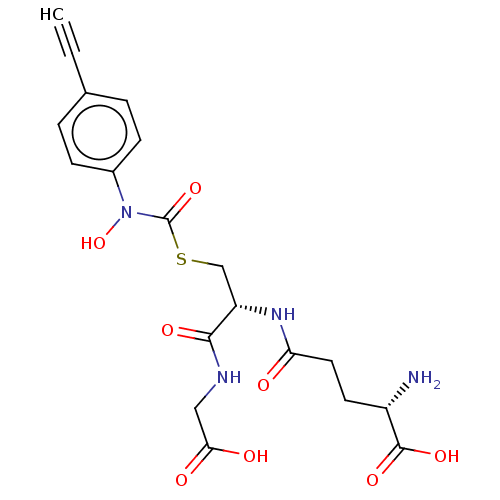 (CHEMBL4450158) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50526942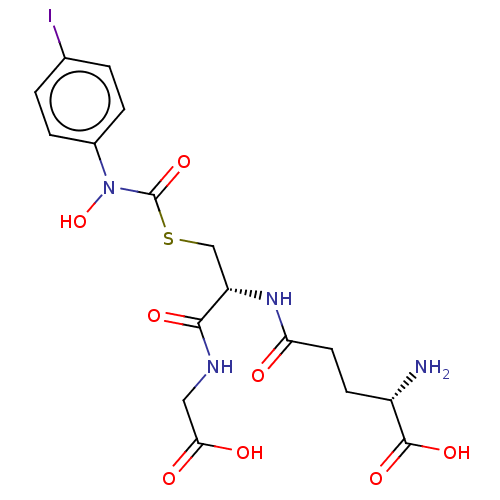 (CHEMBL4438930) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human Glyoxalase-1 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50402202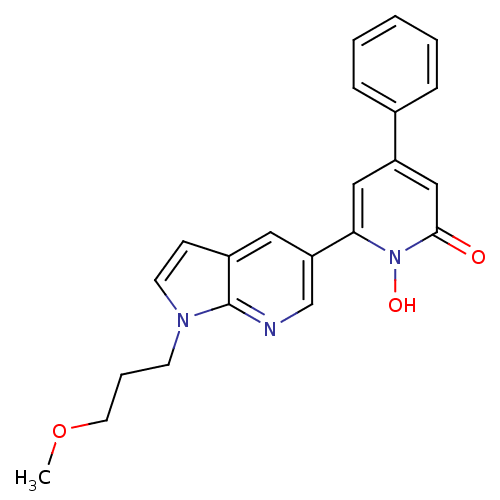 (CHEMBL2203964) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human GLO1 | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092826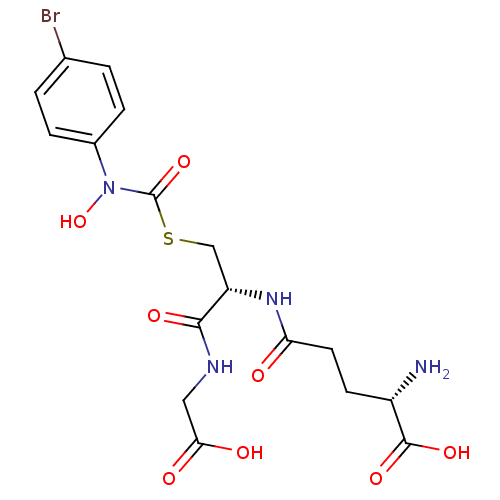 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human glyoxalase 1 | Bioorg Med Chem Lett 25: 4724-7 (2015) Article DOI: 10.1016/j.bmcl.2015.08.055 BindingDB Entry DOI: 10.7270/Q26H4K7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092826 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte Glyoxalase-1 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092825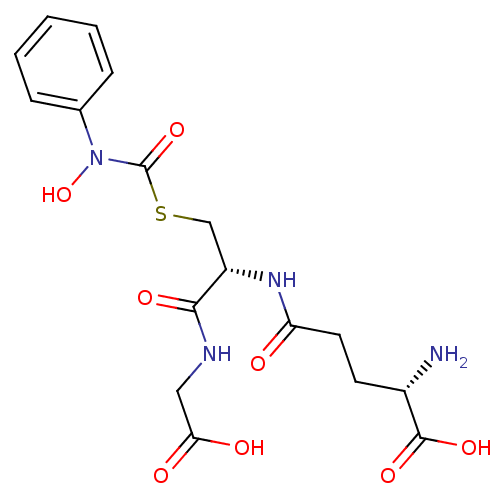 (CHEMBL128935 | S-(N-phenyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human GLO1 | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092826 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039111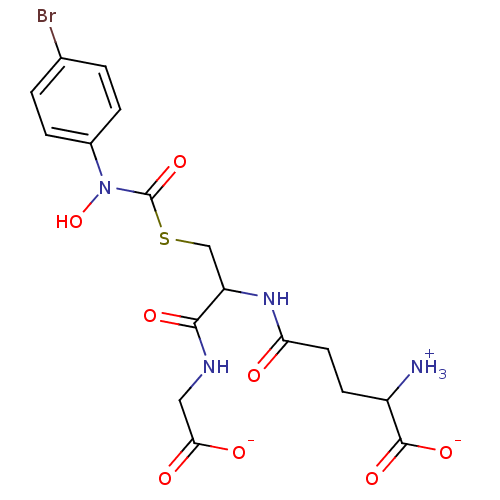 (S-(N-Hydroxy-N-(4-bromophenyl)carbamoyl)glutathion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against human erythrocyte glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092827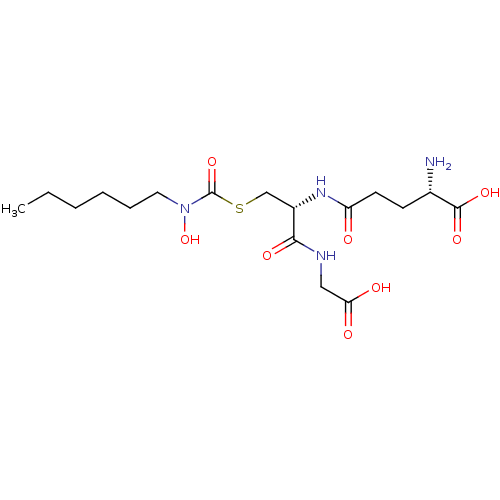 (CHEMBL127840 | S-(N-hexyl-N-hydroxycarbamoyl)gluta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092831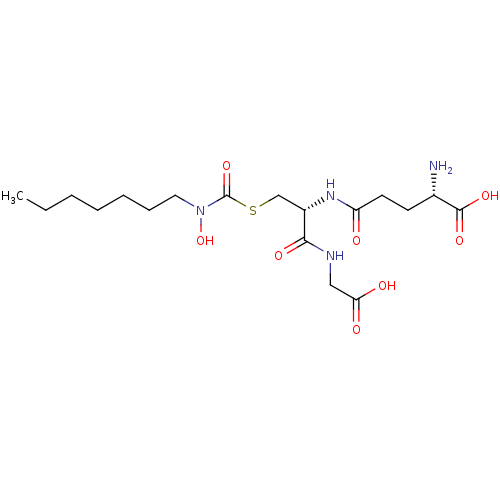 (CHEMBL128836 | S-(N-heptyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50526948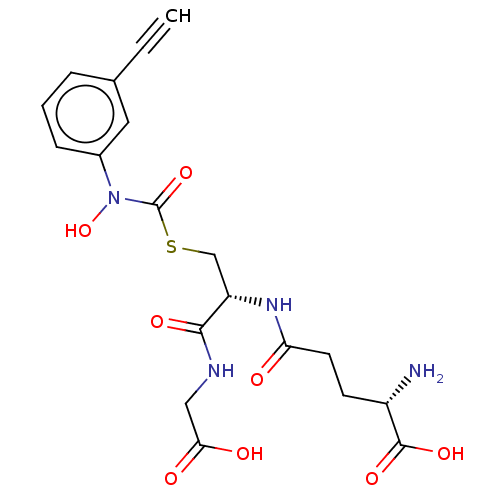 (CHEMBL4559486) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039108 (S-(N-Hydroxy-N-(4-chlorophenyl)carbamoyl)glutathio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against human erythrocyte glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50126957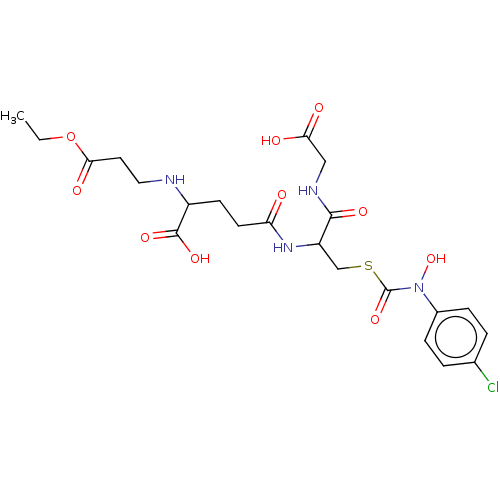 (CHEMBL3629119) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human glyoxalase 1 | Bioorg Med Chem Lett 25: 4724-7 (2015) Article DOI: 10.1016/j.bmcl.2015.08.055 BindingDB Entry DOI: 10.7270/Q26H4K7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092824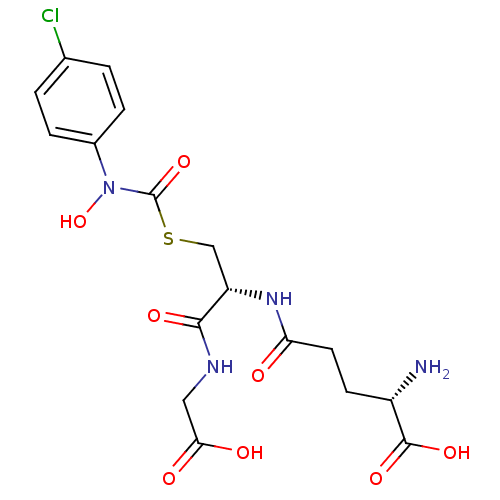 (CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte Glyoxalase-1 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092824 (CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human glyoxalase 1 | Bioorg Med Chem Lett 25: 4724-7 (2015) Article DOI: 10.1016/j.bmcl.2015.08.055 BindingDB Entry DOI: 10.7270/Q26H4K7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092824 (CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Competitive inhibition of GLO1 (unknown origin) | Bioorg Med Chem 22: 3301-8 (2014) Article DOI: 10.1016/j.bmc.2014.04.055 BindingDB Entry DOI: 10.7270/Q29025C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092824 (CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50241121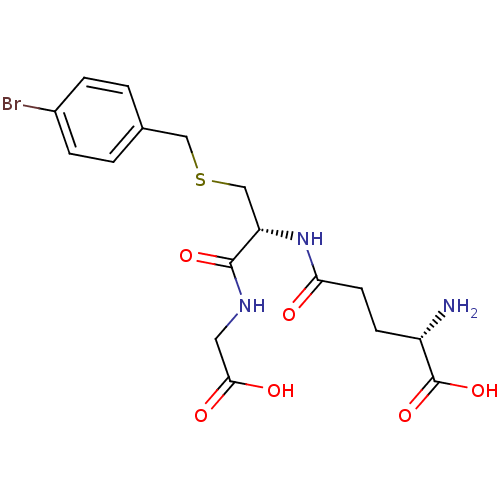 ((S)-5-((R)-3-(4-bromobenzylthio)-1-(carboxymethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of GLO1 (unknown origin) | Bioorg Med Chem 22: 3301-8 (2014) Article DOI: 10.1016/j.bmc.2014.04.055 BindingDB Entry DOI: 10.7270/Q29025C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50126958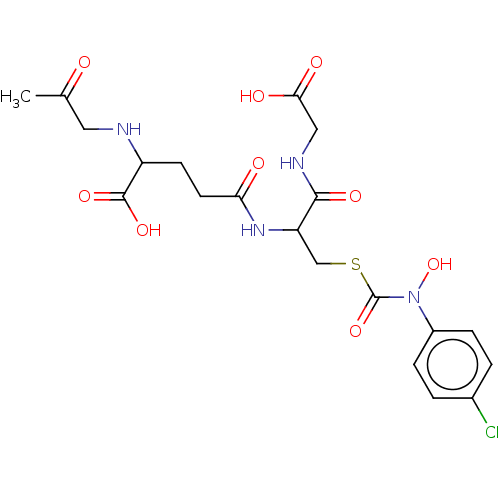 (CHEMBL3629118) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human glyoxalase 1 | Bioorg Med Chem Lett 25: 4724-7 (2015) Article DOI: 10.1016/j.bmcl.2015.08.055 BindingDB Entry DOI: 10.7270/Q26H4K7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092825 (CHEMBL128935 | S-(N-phenyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039113 (S-(N-Hydroxy-N-phenylcarbamoyl)glutathione) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against human erythrocyte glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092825 (CHEMBL128935 | S-(N-phenyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte Glyoxalase-1 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092830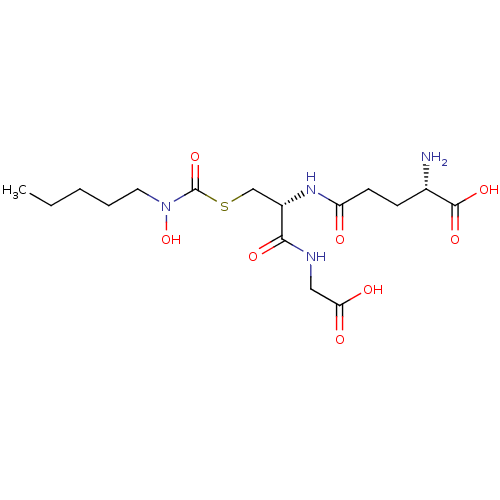 (CHEMBL129965 | S-(N-pentyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50241121 ((S)-5-((R)-3-(4-bromobenzylthio)-1-(carboxymethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of Glyoxalase-1 (unknown origin) using GSH and MGO as substrates preincubated with substrates for 6 mins followed by enzyme addition by sp... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092822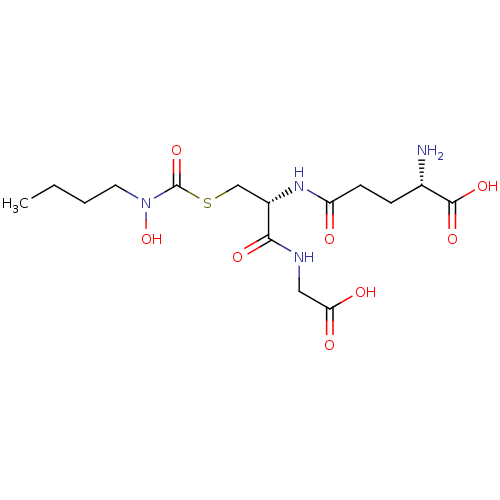 (CHEMBL128447 | S-(N-butyl-N-hydroxycarbamoyl)gluta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517464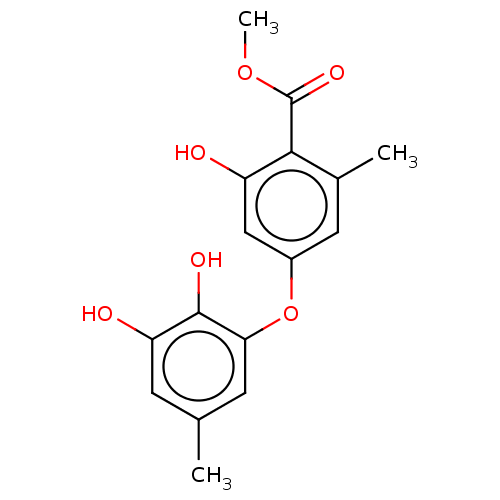 (CHEMBL1234300) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human GLO1 | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50126959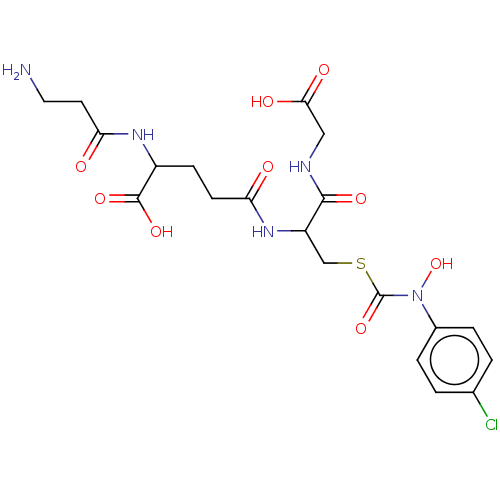 (CHEMBL3629117) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human glyoxalase 1 | Bioorg Med Chem Lett 25: 4724-7 (2015) Article DOI: 10.1016/j.bmcl.2015.08.055 BindingDB Entry DOI: 10.7270/Q26H4K7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092829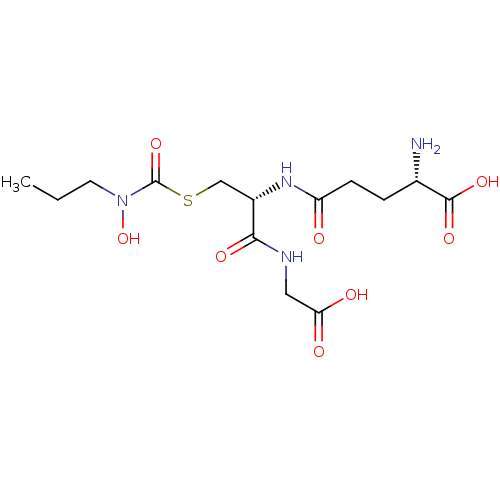 (CHEMBL129597 | S-(N-propyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092832 (CHEMBL128867 | S-(N-ethyl-N-hydroxycarbamoyl)gluta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039111 (S-(N-Hydroxy-N-(4-bromophenyl)carbamoyl)glutathion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against yeast glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092826 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound on yeast glyoxalase I (GlxI) was determined | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039110 (S-(N-Methyl-N-hydroxycarbomyl)ethylglutathione) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against human erythrocyte glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092823 (CHEMBL129435 | S-(N-methyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50069024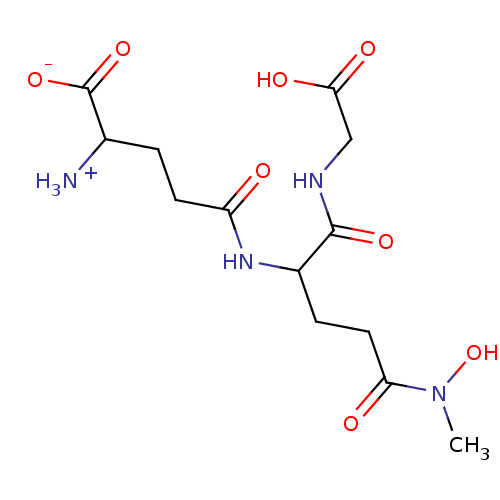 (2-Amino-4-[1-(carboxymethyl-carbamoyl)-3-(hydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of S. cerevisiae glyoxalase-I by using enzymatic assay at each of 6 substrate concentrations between 0.1 mM and... | Bioorg Med Chem Lett 8: 705-10 (1999) BindingDB Entry DOI: 10.7270/Q2KH0MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50336257 (4-(3-fluorobenzylidene)-1,7-bis(4-hydroxy-3-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged glyoxalase 1 expressed in Escherichia coli BL21 (DE3) preincubated for 20 mins by Dixon plot analysis | Bioorg Med Chem 19: 1189-96 (2011) Article DOI: 10.1016/j.bmc.2010.12.039 BindingDB Entry DOI: 10.7270/Q2222V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50336258 (4-(4-fluorobenzylidene)-1,7-bis(4-hydroxy-3-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged glyoxalase 1 expressed in Escherichia coli BL21 (DE3) preincubated for 20 mins by Dixon plot analysis | Bioorg Med Chem 19: 1189-96 (2011) Article DOI: 10.1016/j.bmc.2010.12.039 BindingDB Entry DOI: 10.7270/Q2222V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039108 (S-(N-Hydroxy-N-(4-chlorophenyl)carbamoyl)glutathio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against yeast glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50336259 (4-(5-(3,4-dimethoxyphenyl)-2-(-3-(3,4-dimethoxyphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged glyoxalase 1 expressed in Escherichia coli BL21 (DE3) preincubated for 20 mins by Dixon plot analysis | Bioorg Med Chem 19: 1189-96 (2011) Article DOI: 10.1016/j.bmc.2010.12.039 BindingDB Entry DOI: 10.7270/Q2222V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50336261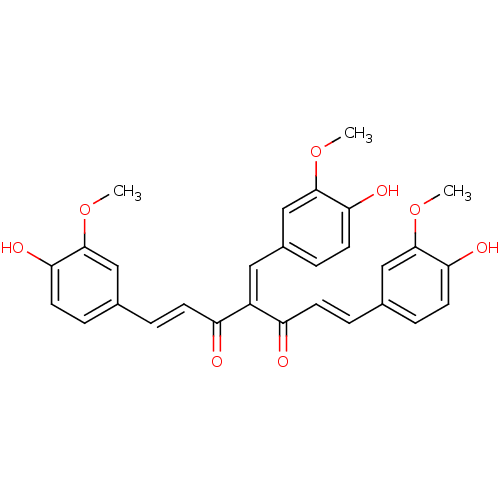 (4-(4-hydroxy-3-methoxybenzylidene)-1,7-bis(4-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged glyoxalase 1 expressed in Escherichia coli BL21 (DE3) preincubated for 20 mins by Dixon plot analysis | Bioorg Med Chem 19: 1189-96 (2011) Article DOI: 10.1016/j.bmc.2010.12.039 BindingDB Entry DOI: 10.7270/Q2222V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092824 (CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound on yeast glyoxalase I (GlxI) was determined | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50241121 ((S)-5-((R)-3-(4-bromobenzylthio)-1-(carboxymethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of S. cerevisiae glyoxalase-I by using enzymatic assay at each of 6 substrate concentrations between 0.1 mM and... | Bioorg Med Chem Lett 8: 705-10 (1999) BindingDB Entry DOI: 10.7270/Q2KH0MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50241121 ((S)-5-((R)-3-(4-bromobenzylthio)-1-(carboxymethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uniroyal Chemical Co., Inc. Curated by ChEMBL | Assay Description Inhibition constant of compound against binding of Yeast Glyoxalase I | J Med Chem 31: 1396-406 (1988) BindingDB Entry DOI: 10.7270/Q2P55QQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50336251 (1,7-bis(4-fluorophenyl)-5-hydroxyhepta-1,4,6-trien...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged glyoxalase 1 expressed in Escherichia coli BL21 (DE3) preincubated for 20 mins by Dixon plot analysis | Bioorg Med Chem 19: 1189-96 (2011) Article DOI: 10.1016/j.bmc.2010.12.039 BindingDB Entry DOI: 10.7270/Q2222V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50336256 (4-(3,4-dimethoxybenzylidene)-1,7-bis(4-hydroxy-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged glyoxalase 1 expressed in Escherichia coli BL21 (DE3) preincubated for 20 mins by Dixon plot analysis | Bioorg Med Chem 19: 1189-96 (2011) Article DOI: 10.1016/j.bmc.2010.12.039 BindingDB Entry DOI: 10.7270/Q2222V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 307 total ) | Next | Last >> |