

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514126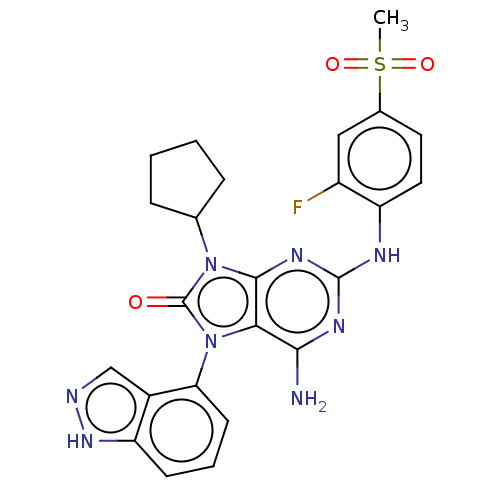 (US11052091, Example 5-1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354414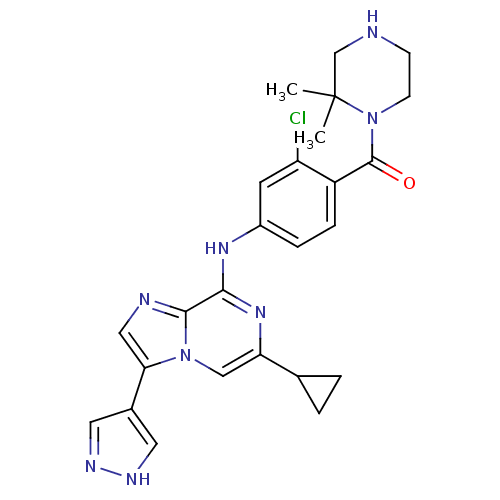 (CHEMBL1836842) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BRK pretreated for 30 mins by microplate reader | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514134 (US11052091, Example 5-16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581757 (US11512087, Example 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514135 (US11052091, Example 5-17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354416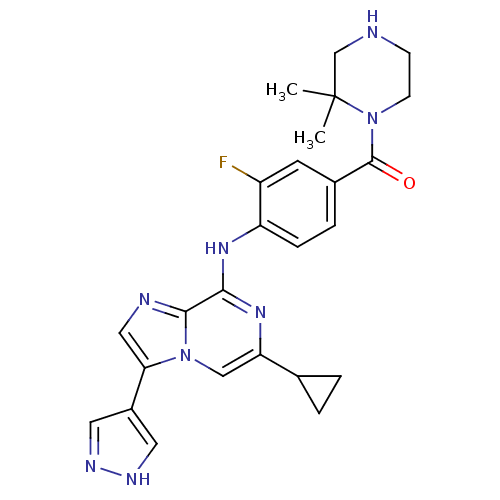 (CHEMBL1836863) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BRK pretreated for 30 mins by microplate reader | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514137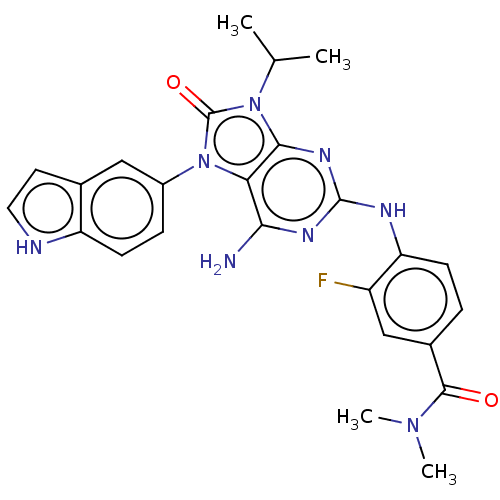 (US11052091, Example 5-19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514136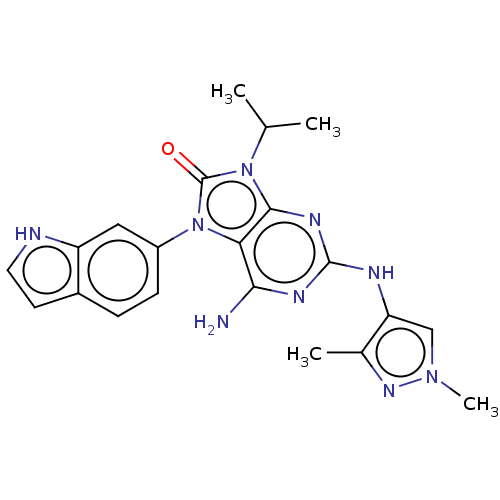 (US11052091, Example 5-18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354415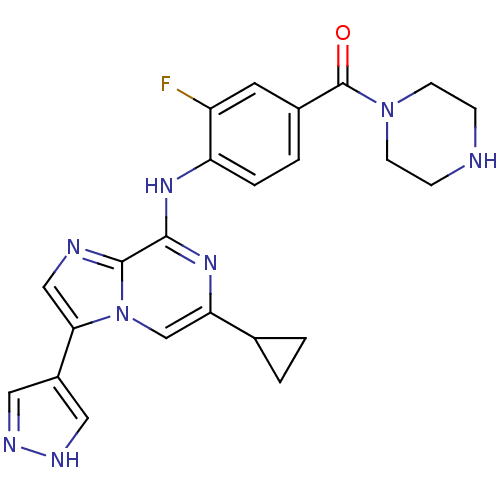 (CHEMBL1836854) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BRK pretreated for 30 mins by microplate reader | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354432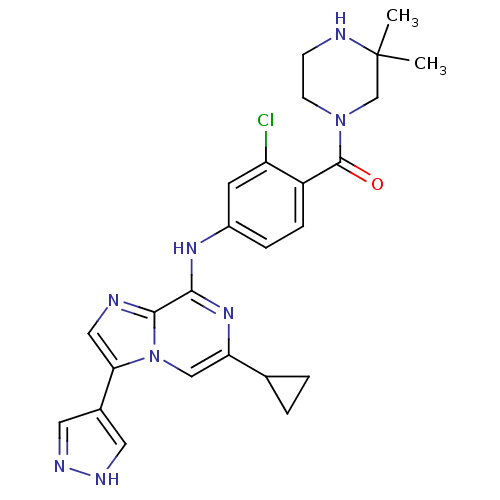 (CHEMBL1836844) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BRK pretreated for 30 mins by microplate reader | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354412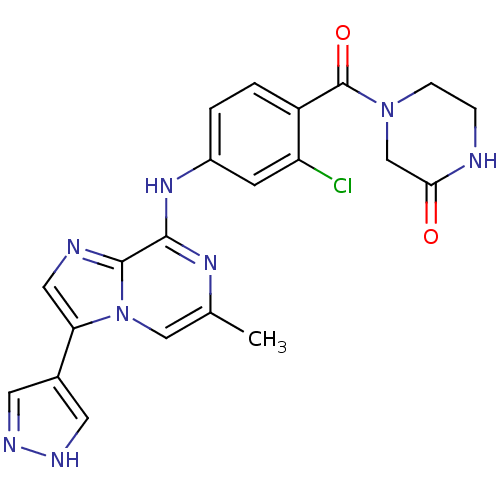 (CHEMBL1836806) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BRK pretreated for 30 mins by microplate reader | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001733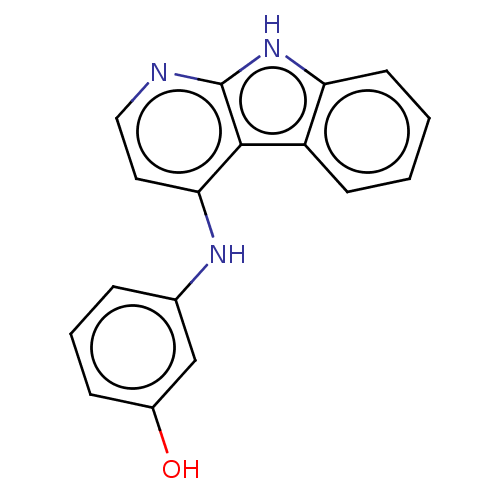 (CHEMBL3133821) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581831 (US11512087, Example 13-1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581837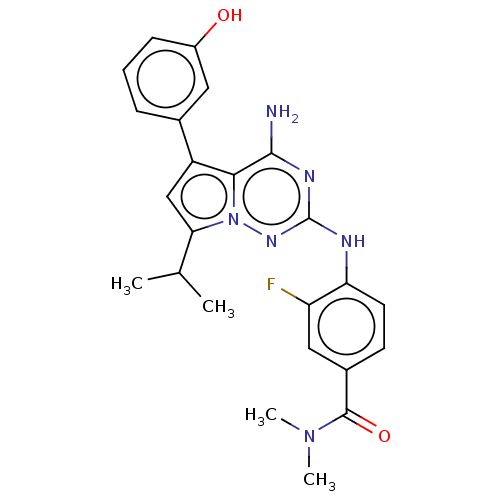 (US11512087, Example 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581838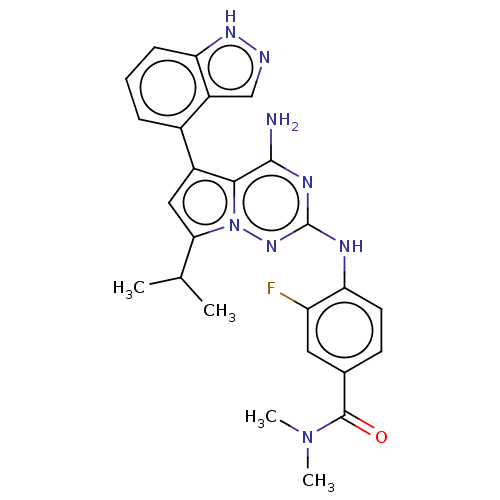 (US11512087, Example 26-1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581832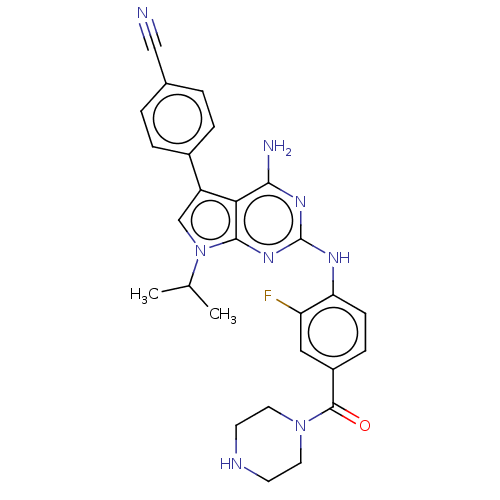 (US11512087, Example 13-9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001734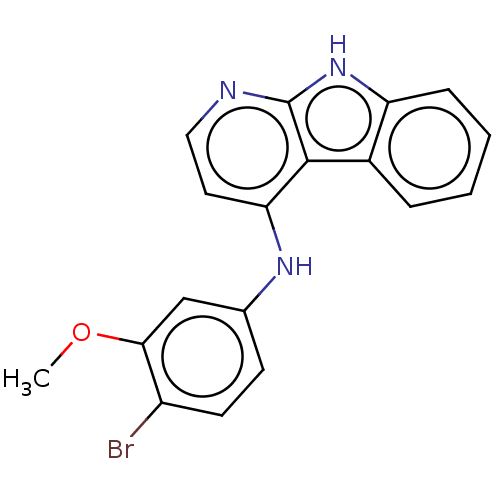 (CHEMBL3238103) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001735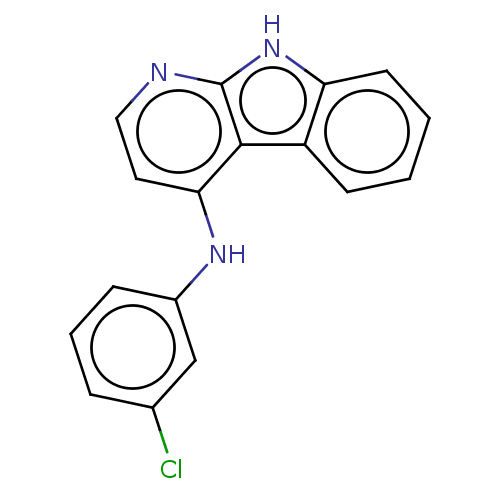 (CHEMBL3133822) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354429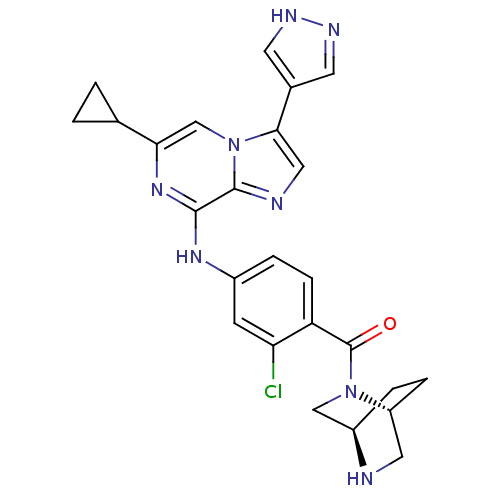 (CHEMBL1836846) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BRK pretreated for 30 mins by microplate reader | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514127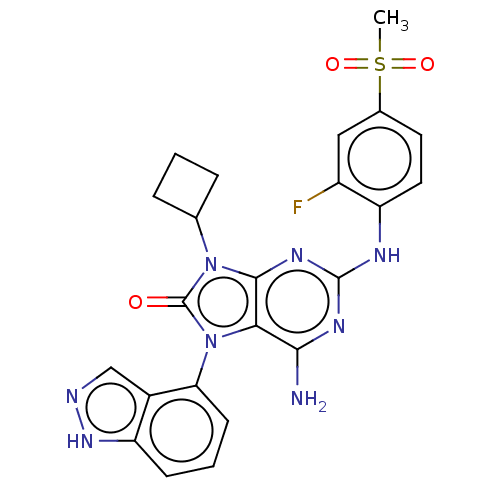 (US11052091, Example 5-2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514138 (US11052091, Example 5-21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354414 (CHEMBL1836842) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phosphorylated SAM68 in 293 WT-PTK6 cells after 3 hrs | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514124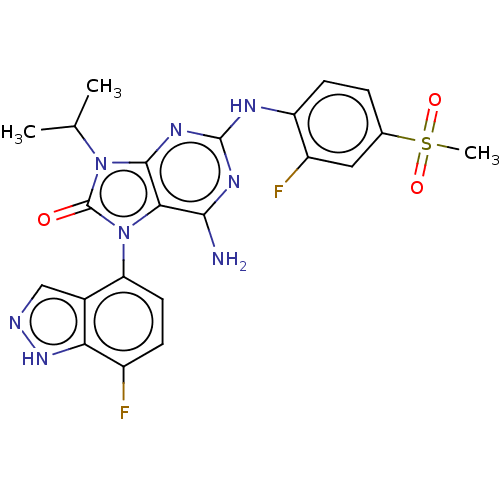 (US11052091, Example 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581795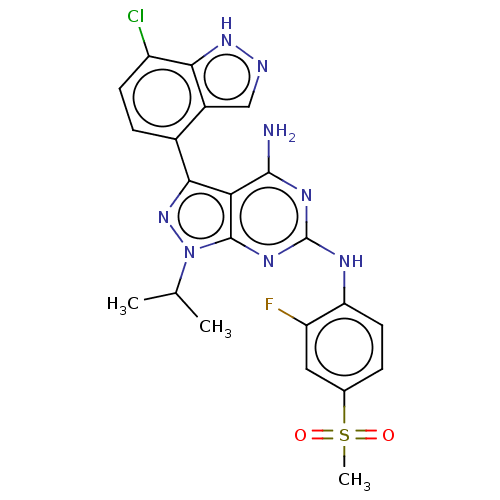 (US11512087, Example 4-1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581830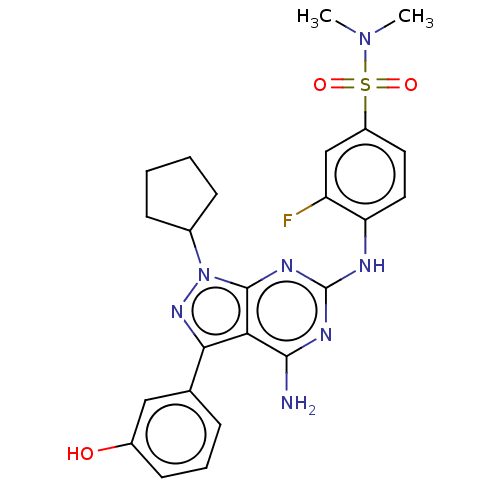 (US11512087, Example 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Silesia in Katowice Curated by ChEMBL | Assay Description Inhibition of BRK (unknown origin) preincubated for 10 mins followed by substrate addition and measured after 1 hr by ADP-Glo luminescence assay | Eur J Med Chem 163: 610-625 (2019) Article DOI: 10.1016/j.ejmech.2018.12.012 BindingDB Entry DOI: 10.7270/Q29P353V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Silesia in Katowice Curated by ChEMBL | Assay Description Inhibition of BRK (unknown origin) preincubated for 10 mins followed by substrate addition and measured after 1 hr by ADP-Glo luminescence assay | Eur J Med Chem 163: 610-625 (2019) Article DOI: 10.1016/j.ejmech.2018.12.012 BindingDB Entry DOI: 10.7270/Q29P353V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581780 (US11512087, Example 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50001732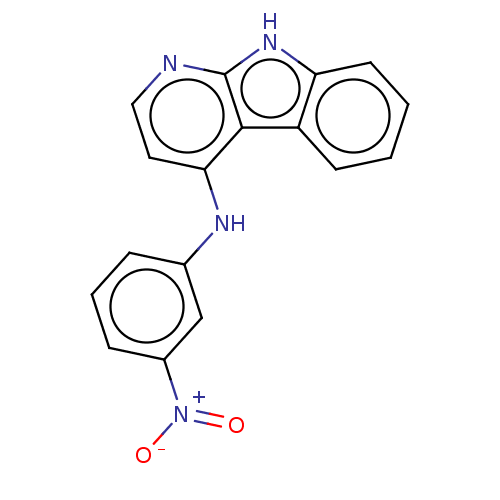 (CHEMBL3238097) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Brk (unknown origin) using [gamma-33P]-ATP after 60 mins by scintillation counting | Bioorg Med Chem Lett 24: 1948-51 (2014) Article DOI: 10.1016/j.bmcl.2014.03.002 BindingDB Entry DOI: 10.7270/Q2TM7CN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514128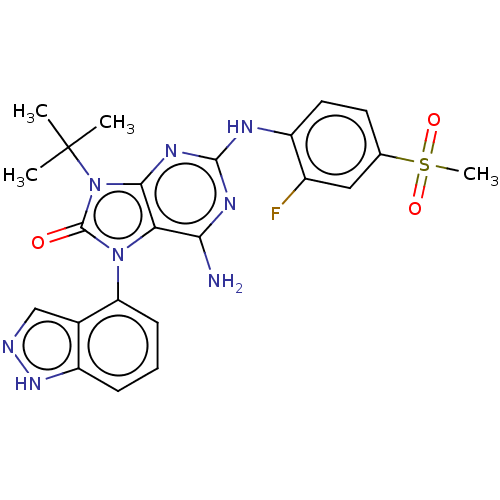 (US11052091, Example 5-3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514129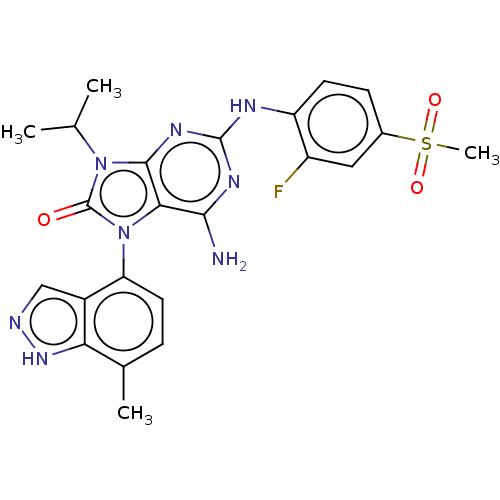 (US11052091, Example 5-4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354455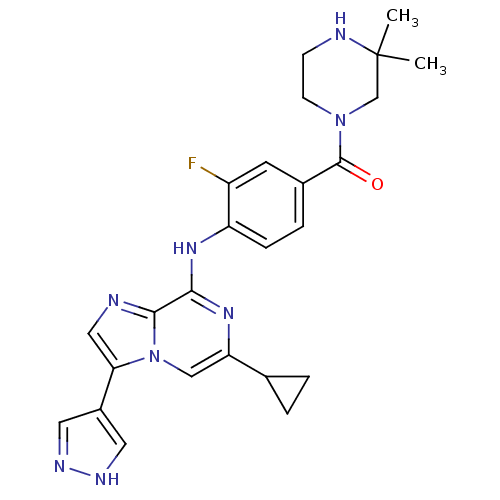 (CHEMBL1836865) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BRK pretreated for 30 mins by microplate reader | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354420 (CHEMBL1836755) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BRK pretreated for 30 mins by microplate reader | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581829 (US11512087, Example 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514130 (US11052091, Example 5-5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514139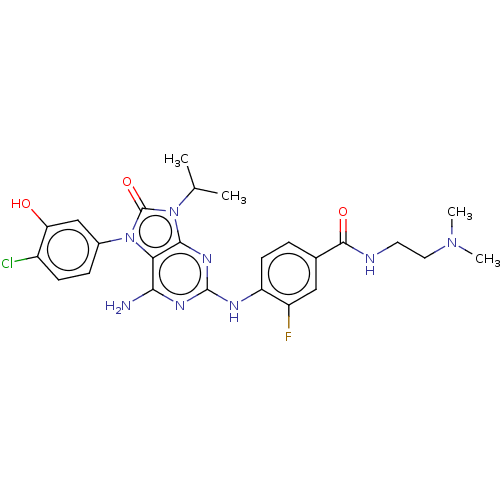 (US11052091, Example 5-22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354455 (CHEMBL1836865) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phosphorylated SAM68 in 293 WT-PTK6 cells after 3 hrs | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354431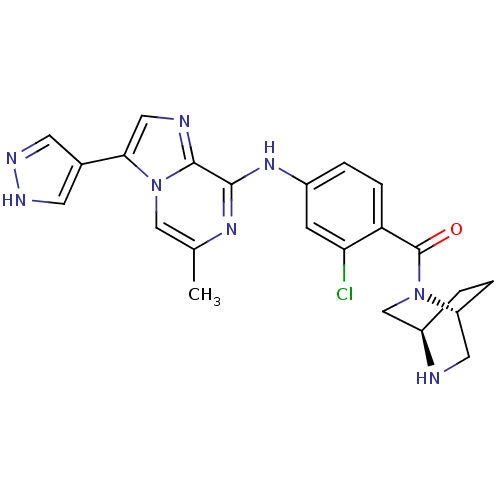 (CHEMBL1836840) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BRK pretreated for 30 mins by microplate reader | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM514121 (US11052091, Example 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354408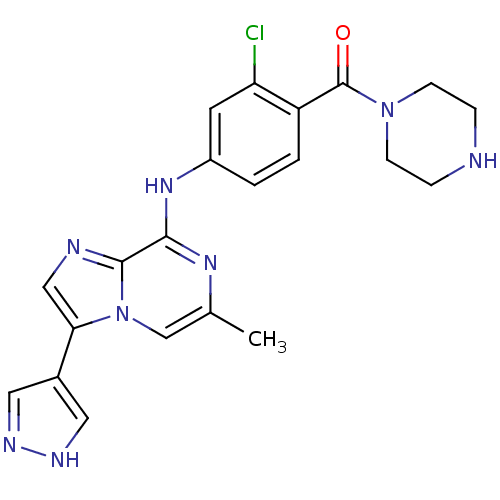 (CHEMBL1836867) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BRK pretreated for 30 mins by microplate reader | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354415 (CHEMBL1836854) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phosphorylated SAM68 in 293 WT-PTK6 cells after 3 hrs | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354416 (CHEMBL1836863) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phosphorylated SAM68 in 293 WT-PTK6 cells after 3 hrs | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354424 (CHEMBL1836802) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BRK pretreated for 30 mins by microplate reader | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581828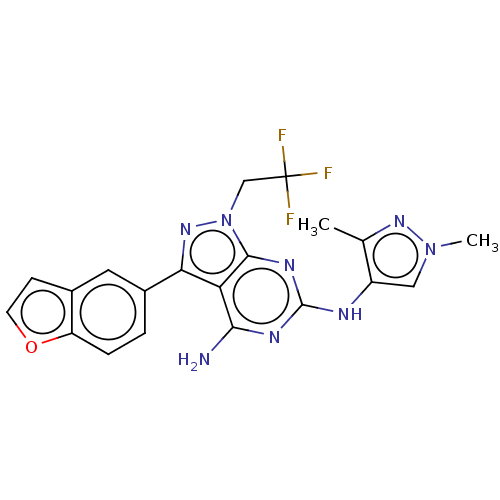 (US11512087, Example 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM581835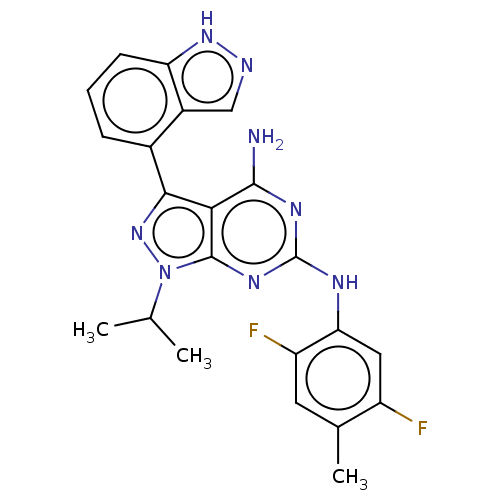 (US11512087, Example 18-1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Measurement of an inhibitory activity on Brk enzyme was performed by using LanthaScreen (registered trademark) system (Invitrogen) in accordance with... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VT1WXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 6 (Homo sapiens (Human)) | BDBM50354409 (CHEMBL1836805) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BRK pretreated for 30 mins by microplate reader | Bioorg Med Chem Lett 21: 5870-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.101 BindingDB Entry DOI: 10.7270/Q2PR7WCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 461 total ) | Next | Last >> |