 Found 15 hits of affinity data for UniProtKB/TrEMBL: Q93086
Found 15 hits of affinity data for UniProtKB/TrEMBL: Q93086 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50598317

(CHEMBL5208562)Show SMILES [O-][N+](=O)c1ccc(NC(=S)NC(=O)c2ccc3OCOc3c2)cc1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50596631
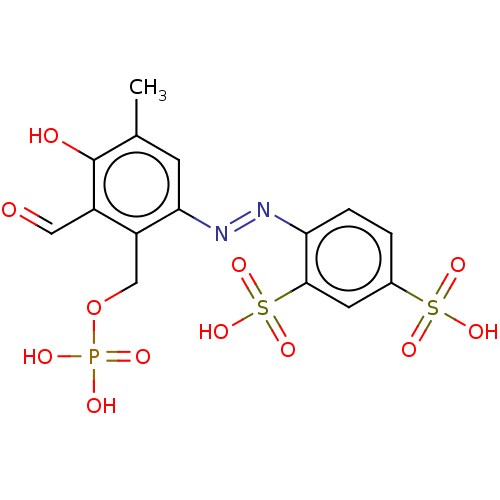
(CHEMBL1615626)Show SMILES Cc1cc(\N=N\c2ccc(cc2S(O)(=O)=O)S(O)(=O)=O)c(COP(O)(O)=O)c(C=O)c1O | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114162
BindingDB Entry DOI: 10.7270/Q2S46X0K |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50540409
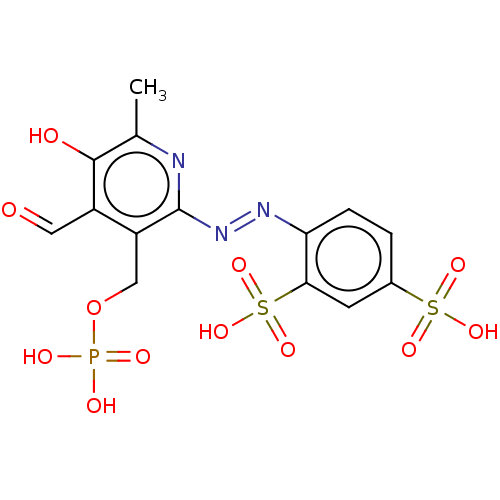
(CHEBI:34941 | CHEMBL69234)Show SMILES Cc1nc(\N=N\c2ccc(cc2S(O)(=O)=O)S(O)(=O)=O)c(COP(O)(O)=O)c(C=O)c1O Show InChI InChI=1S/C14H14N3O12PS2/c1-7-13(19)9(5-18)10(6-29-30(20,21)22)14(15-7)17-16-11-3-2-8(31(23,24)25)4-12(11)32(26,27)28/h2-5,19H,6H2,1H3,(H2,20,21,22)(H,23,24,25)(H,26,27,28)/b17-16+ | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50596630

(CHEMBL5172961)Show SMILES Cc1ccc(NC(=S)NC(=O)C23CC4CC(CC(C4)C2)C3)c(Br)c1 |TLB:18:17:20:14.13.12,18:13:20:17.19.16,THB:16:17:20.15.14:12,16:15:17.19.18:12| | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114162
BindingDB Entry DOI: 10.7270/Q2S46X0K |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50499490
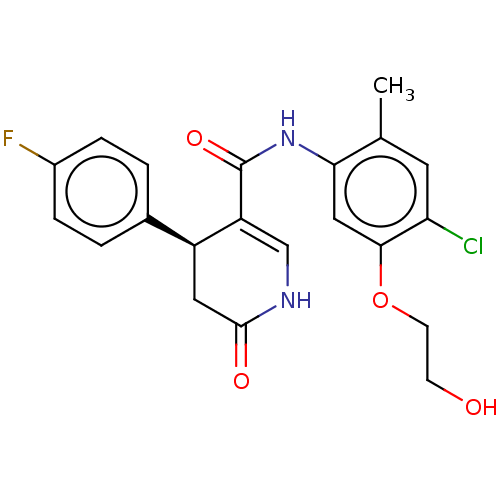
(CHEMBL3741992)Show SMILES Cc1cc(Cl)c(OCCO)cc1NC(=O)C1=CNC(=O)C[C@H]1c1ccc(F)cc1 |r,t:16| Show InChI InChI=1S/C21H20ClFN2O4/c1-12-8-17(22)19(29-7-6-26)10-18(12)25-21(28)16-11-24-20(27)9-15(16)13-2-4-14(23)5-3-13/h2-5,8,10-11,15,26H,6-7,9H2,1H3,(H,24,27)(H,25,28)/t15-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of P2X5R (unknown origin) |
J Med Chem 58: 8413-26 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00365
BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50415563
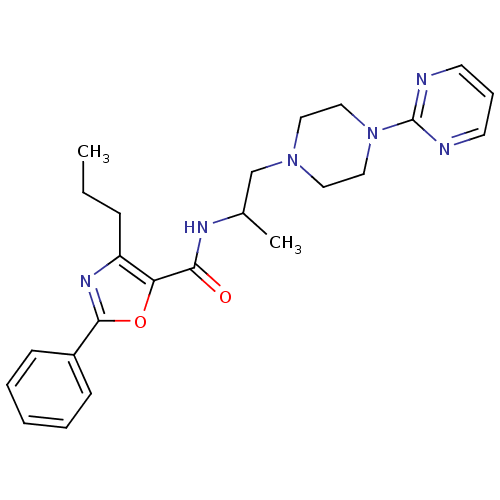
(CHEMBL605325)Show SMILES CCCc1nc(oc1C(=O)NC(C)CN1CCN(CC1)c1ncccn1)-c1ccccc1 Show InChI InChI=1S/C24H30N6O2/c1-3-8-20-21(32-23(28-20)19-9-5-4-6-10-19)22(31)27-18(2)17-29-13-15-30(16-14-29)24-25-11-7-12-26-24/h4-7,9-12,18H,3,8,13-17H2,1-2H3,(H,27,31) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X5 receptor |
Bioorg Med Chem Lett 20: 1031-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.044
BindingDB Entry DOI: 10.7270/Q22V2HCR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50415550
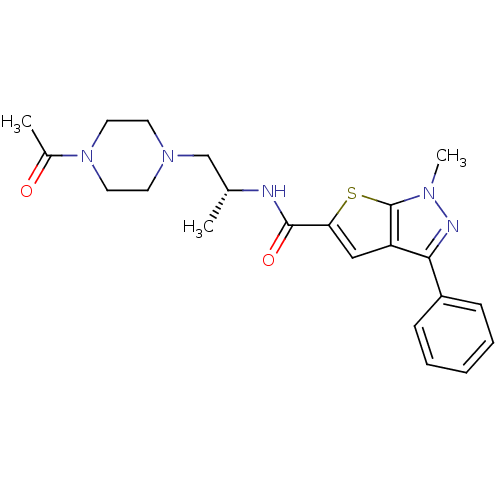
(CHEMBL599760 | Ro-85)Show SMILES C[C@H](CN1CCN(CC1)C(C)=O)NC(=O)c1cc2c(nn(C)c2s1)-c1ccccc1 |r| Show InChI InChI=1S/C22H27N5O2S/c1-15(14-26-9-11-27(12-10-26)16(2)28)23-21(29)19-13-18-20(17-7-5-4-6-8-17)24-25(3)22(18)30-19/h4-8,13,15H,9-12,14H2,1-3H3,(H,23,29)/t15-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X5 receptor |
Bioorg Med Chem Lett 20: 1031-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.044
BindingDB Entry DOI: 10.7270/Q22V2HCR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50257829
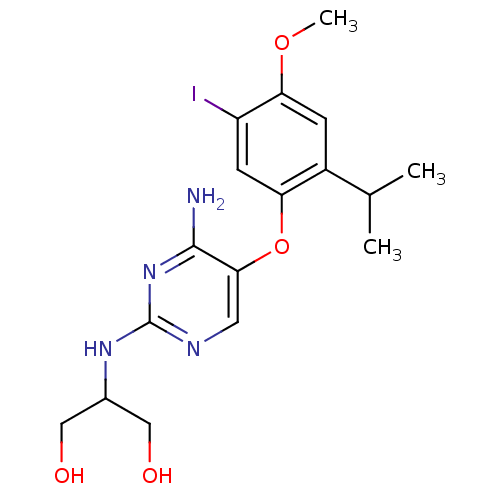
(2-(4-amino-5-(5-iodo-2-isopropyl-4-methoxyphenoxy)...)Show InChI InChI=1S/C17H23IN4O4/c1-9(2)11-4-14(25-3)12(18)5-13(11)26-15-6-20-17(22-16(15)19)21-10(7-23)8-24/h4-6,9-10,23-24H,7-8H2,1-3H3,(H3,19,20,21,22) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X5 receptor up to 10 uM |
Bioorg Med Chem Lett 19: 1632-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.097
BindingDB Entry DOI: 10.7270/Q2DN44ZM |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50257636
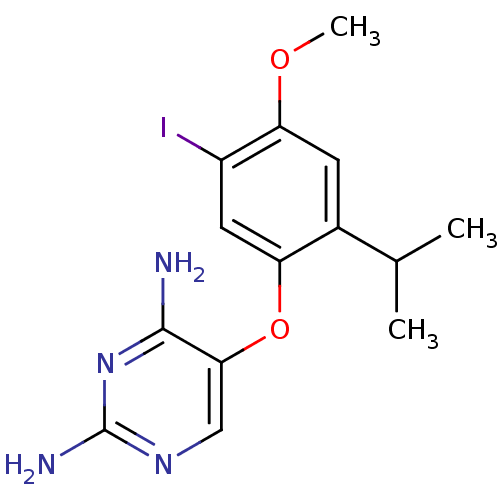
(5-(5-iodo-2-isopropyl-4-methoxyphenoxy)pyrimidine-...)Show InChI InChI=1S/C14H17IN4O2/c1-7(2)8-4-11(20-3)9(15)5-10(8)21-12-6-18-14(17)19-13(12)16/h4-7H,1-3H3,(H4,16,17,18,19) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of P2X5 receptor |
Bioorg Med Chem Lett 19: 1628-31 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.003
BindingDB Entry DOI: 10.7270/Q29023NX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50596625
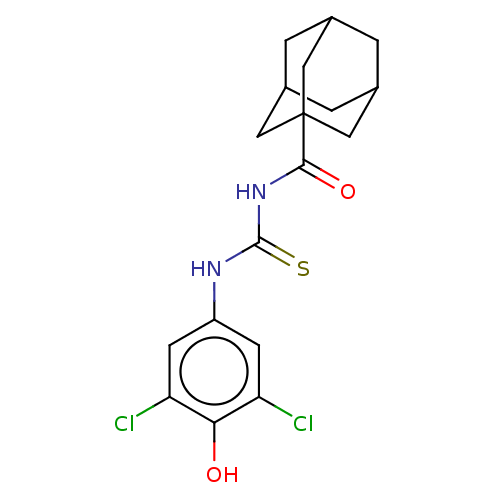
(CHEMBL5174645)Show SMILES Oc1c(Cl)cc(NC(=S)NC(=O)C23CC4CC(CC(C4)C2)C3)cc1Cl |TLB:19:18:21:15.14.13,19:14:21:18.20.17,THB:17:18:21.16.15:13,17:16:18.20.19:13| | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114162
BindingDB Entry DOI: 10.7270/Q2S46X0K |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50179360

(CHEMBL3040216)Show SMILES CC1=CCC(=C\C1=N\C(=O)C1=CC=C\C(C1)=N/C(=O)/N=C1/CC(=CC=C1)C(=O)\N=C1\CC(=CC=C1C)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O |c:4,13,24,26,34,36,45,72,t:1,11| Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-11,15-16,20-24H,12-14,17-19H2,1-2H3,(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)/b52-31+,53-32+,54-37+,55-38+,56-39-,57-40- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50179360

(CHEMBL3040216)Show SMILES CC1=CCC(=C\C1=N\C(=O)C1=CC=C\C(C1)=N/C(=O)/N=C1/CC(=CC=C1)C(=O)\N=C1\CC(=CC=C1C)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O |c:4,13,24,26,34,36,45,72,t:1,11| Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-11,15-16,20-24H,12-14,17-19H2,1-2H3,(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)/b52-31+,53-32+,54-37+,55-38+,56-39-,57-40- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114162
BindingDB Entry DOI: 10.7270/Q2S46X0K |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50596629
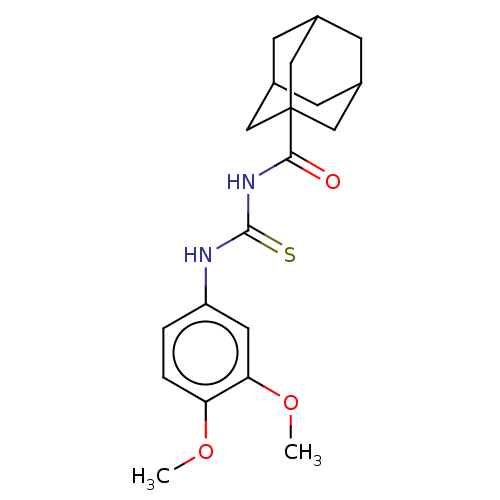
(CHEMBL5200337)Show SMILES COc1ccc(NC(=S)NC(=O)C23CC4CC(CC(C4)C2)C3)cc1OC |TLB:19:18:21:15.14.13,19:14:21:18.20.17,THB:17:18:21.16.15:13,17:16:18.20.19:13| | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114162
BindingDB Entry DOI: 10.7270/Q2S46X0K |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50598315

(CHEMBL5186938) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114491
BindingDB Entry DOI: 10.7270/Q2BG2T1P |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 5
(Homo sapiens (Human)) | BDBM50118219
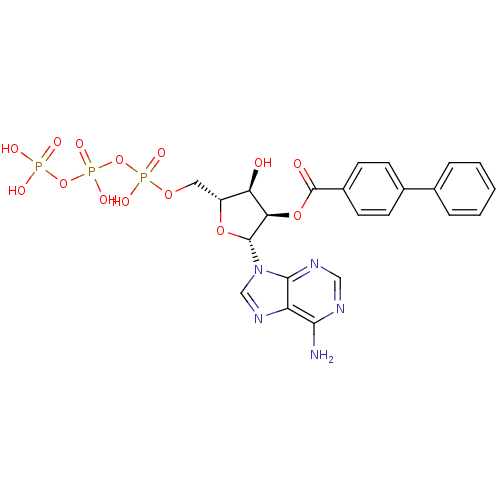
(Bz-ATP | CHEMBL339386)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1OC(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C23H24N5O14P3/c24-20-17-21(26-11-25-20)28(12-27-17)22-19(40-23(30)15-8-6-14(7-9-15)13-4-2-1-3-5-13)18(29)16(39-22)10-38-44(34,35)42-45(36,37)41-43(31,32)33/h1-9,11-12,16,18-19,22,29H,10H2,(H,34,35)(H,36,37)(H2,24,25,26)(H2,31,32,33)/t16-,18-,19-,22-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes
Curated by ChEMBL
| Assay Description
The compound was evaluated for antagonist activity against recombinant human P2X purinoceptor 5 (P2X5) |
J Med Chem 45: 4057-93 (2002)
BindingDB Entry DOI: 10.7270/Q2VX0H71 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data