

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50087247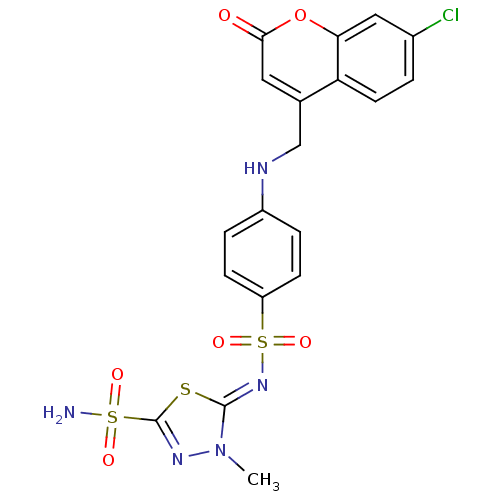 (5-{4-[(7-Chloro-2-oxo-2H-chromen-4-ylmethyl)-amino...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibition of bovine Carbonic anhydrase IV determined by esterase method | Bioorg Med Chem Lett 10: 673-6 (2000) BindingDB Entry DOI: 10.7270/Q2RV0P61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50080684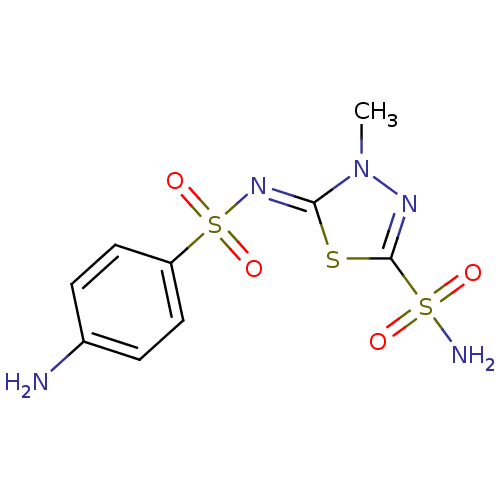 (5-(4-aminophenylsulfonylimino)-4-methyl-4,5-dihydr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes | J Med Chem 42: 3690-700 (1999) Article DOI: 10.1021/jm9901879 BindingDB Entry DOI: 10.7270/Q26Q1XXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50114157 ((R)-4-((8S,10S,12S,14R,15R,17S)-10,13-Dimethyl-3,7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV (CA4), obtained from bovine lung microsomes | Bioorg Med Chem Lett 12: 1551-7 (2002) BindingDB Entry DOI: 10.7270/Q2B56K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50084563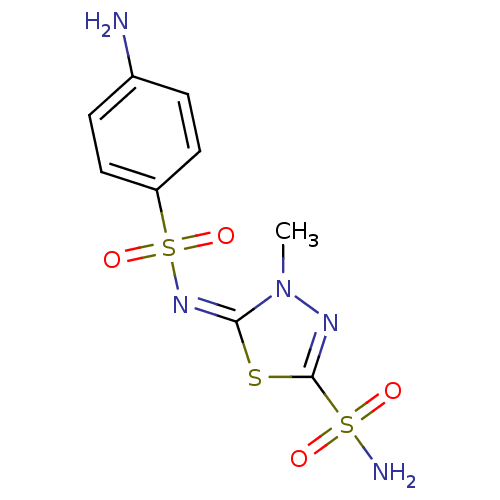 (5-(4-Amino-benzenesulfonylimino)-4-methyl-4,5-dihy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes | J Med Chem 43: 292-300 (2000) BindingDB Entry DOI: 10.7270/Q22806T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50084563 (5-(4-Amino-benzenesulfonylimino)-4-methyl-4,5-dihy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibition of bovine carbonic anhydrase IV from bovine lung microsomes | Bioorg Med Chem Lett 10: 1117-20 (2000) BindingDB Entry DOI: 10.7270/Q2WW7J6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50114120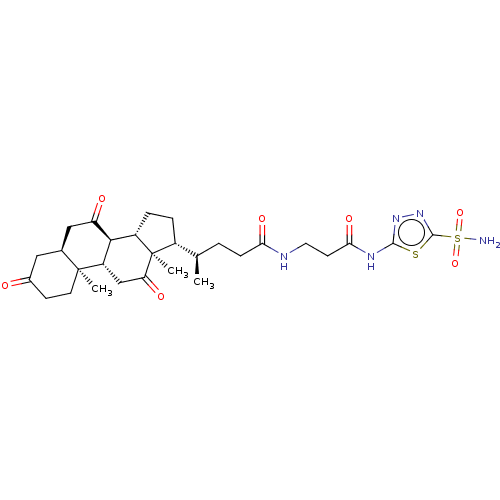 ((R)-4-((2R,6S,7S,8S,10R,12S)-10,13-Dimethyl-3,7,12...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV (CA4), obtained from bovine lung microsomes | Bioorg Med Chem Lett 12: 1551-7 (2002) BindingDB Entry DOI: 10.7270/Q2B56K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50114129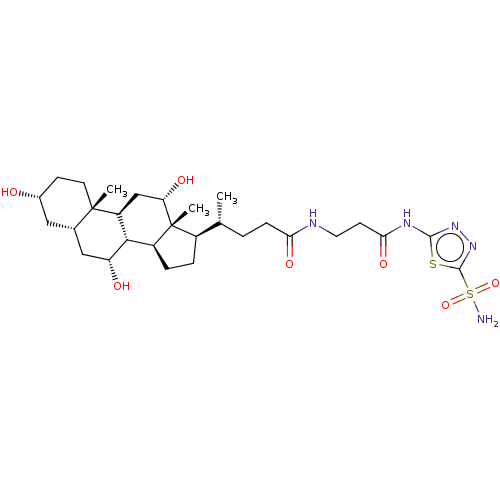 (4-((6R,7S,8S,10R,11S,12S,14S,15R)-3,12-Di(R)-hydro...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV (CA4), obtained from bovine lung microsomes | Bioorg Med Chem Lett 12: 1551-7 (2002) BindingDB Entry DOI: 10.7270/Q2B56K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50114147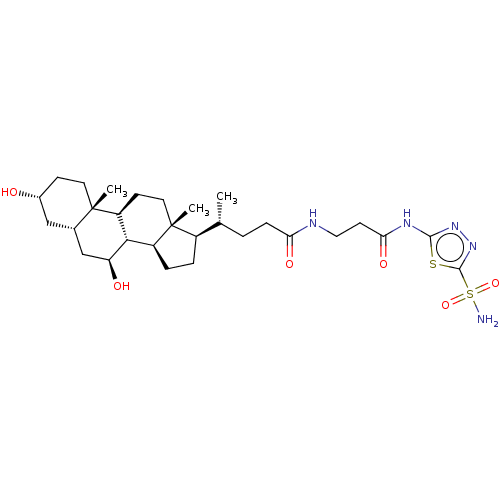 ((R)-4-((5S,6R,8S,9S,12S,14R,17S)-7-Hydroxy-3-(R)-h...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV (CA4), obtained from bovine lung microsomes | Bioorg Med Chem Lett 12: 1551-7 (2002) BindingDB Entry DOI: 10.7270/Q2B56K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50088778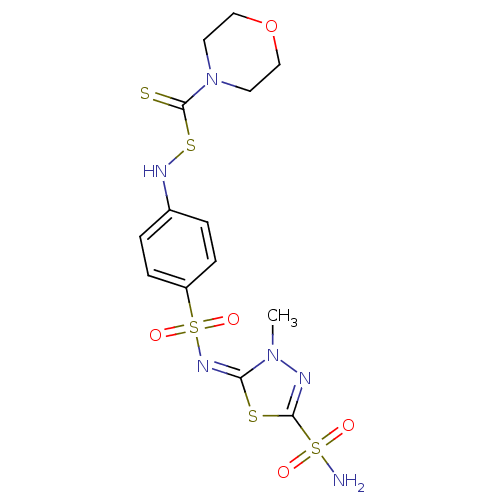 (CHEMBL275565 | N,N-dialkylthio-carbonylsulfenylami...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibition of bovine carbonic anhydrase IV from bovine lung microsomes | Bioorg Med Chem Lett 10: 1117-20 (2000) BindingDB Entry DOI: 10.7270/Q2WW7J6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11631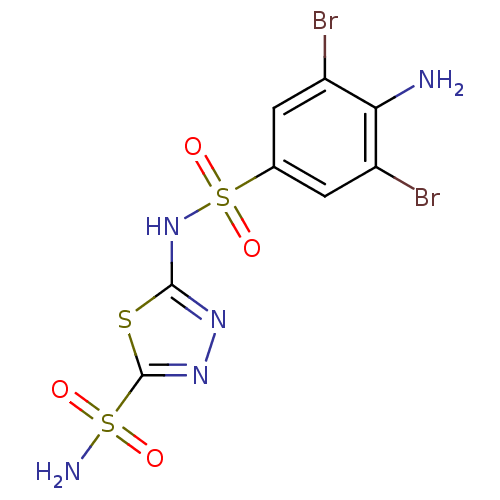 (2-N-(4-amino-3,5-dibromobenzene)-1,3,4-thiadiazole...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11626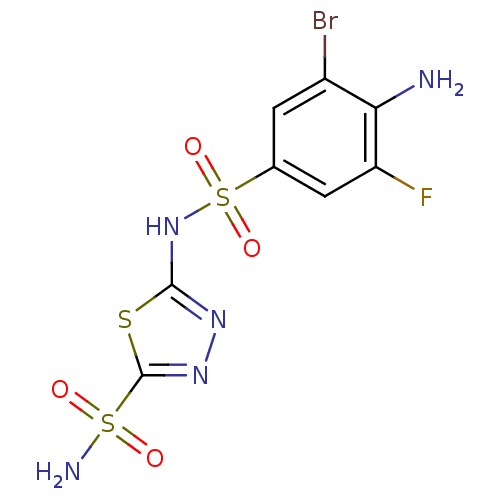 (β-CA inhibitor, 2 | 2-N-(4-amino-3-bromo-5-fl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11628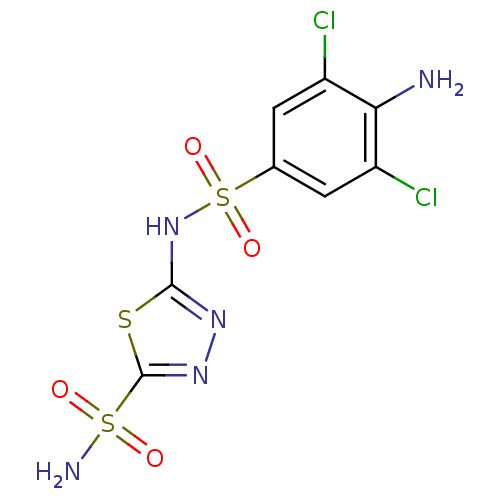 (2-N-(4-amino-3,5-dichlorobenzene)-1,3,4-thiadiazol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50114130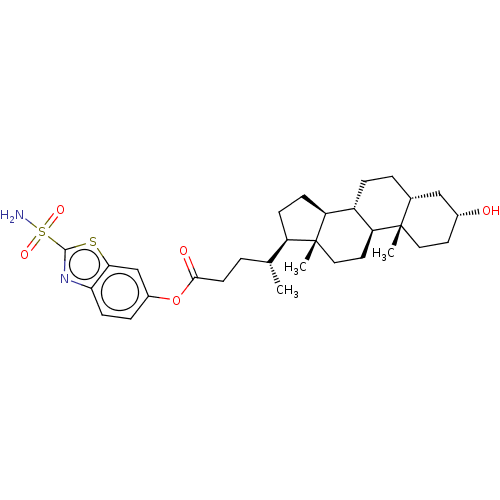 ((1R,2R)-4-((8S,9S,12R,14R,17S)-3-(R)-Hydroxy-10,13...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV (CA4), obtained from bovine lung microsomes | Bioorg Med Chem Lett 12: 1551-7 (2002) BindingDB Entry DOI: 10.7270/Q2B56K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50114193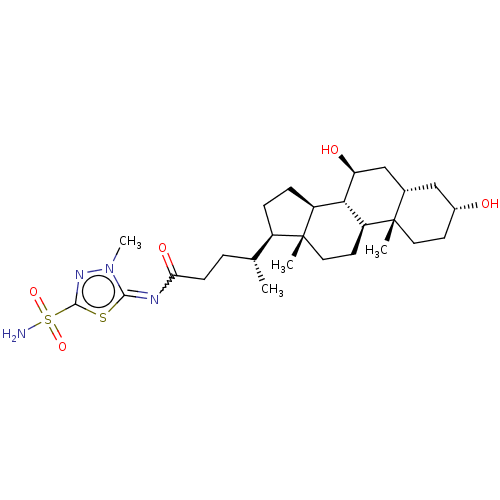 ((R)-4-((7S,8S,9S,10R,12R,14S,17R)-3,7-Dihydroxy-10...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV (CA4), obtained from bovine lung microsomes | Bioorg Med Chem Lett 12: 1551-7 (2002) BindingDB Entry DOI: 10.7270/Q2B56K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11629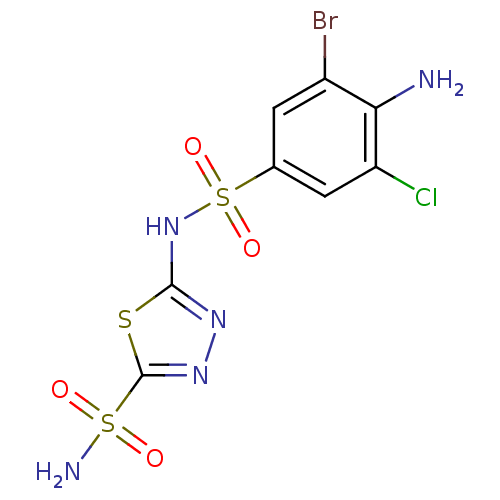 (2-N-(4-amino-3-bromo-5-chlorobenzene)-1,3,4-thiadi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11625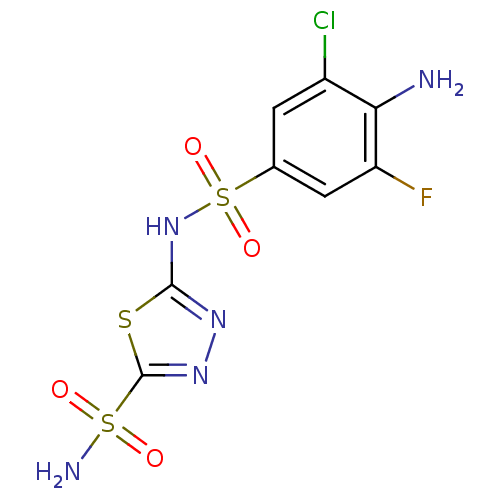 (2-N-(4-amino-3-chloro-5-fluorobenzene)-1,3,4-thiad...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14722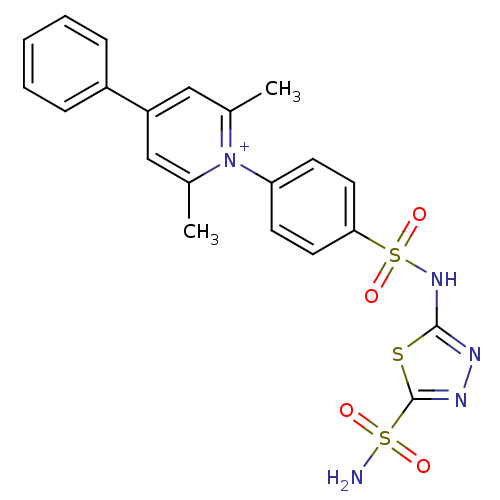 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -48.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
Università degli Studi di Firenze | Assay Description The initial rates of 4-nitrophenylacetate hydrolysis catalysed by different CA isozymes were monitored spectrophotometrically at 400nm with a Cary 3 ... | J Enzyme Inhib Med Chem 19: 269-73 (2004) Article DOI: 10.1080/14756360410001689559 BindingDB Entry DOI: 10.7270/Q20C4TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14722 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2/3/4/6 (Bos taurus-Bos taurus (Cattle)-Bos taurus (bovine)) | BDBM50070606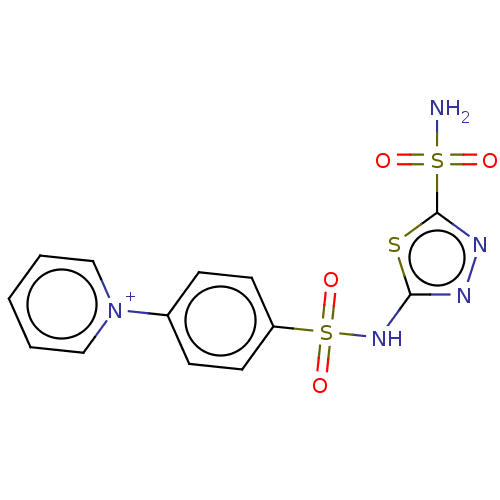 (CHEMBL3408993) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi Curated by ChEMBL | Assay Description Inhibition of bovine carbonic anhydrase 5 | Eur J Med Chem 92: 156-77 (2015) Article DOI: 10.1016/j.ejmech.2014.12.035 BindingDB Entry DOI: 10.7270/Q23B61TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14718 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11627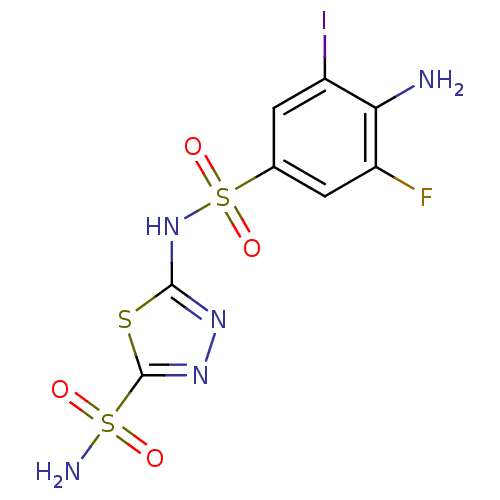 (β-CA inhibitor, 3 | 2-N-(4-amino-3-fluoro-5-i...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50114213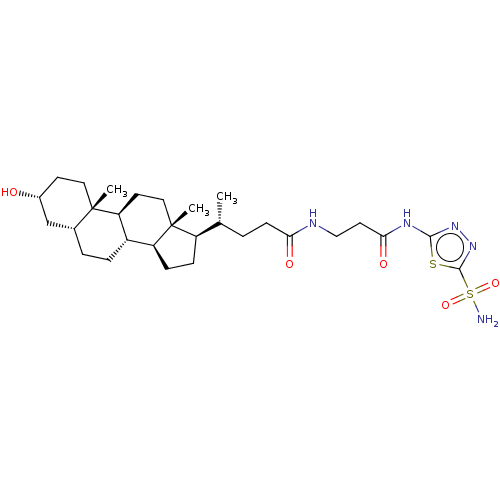 ((1R,5R)-4-((5S,8S,9R,11R,17S)-3-Hydroxy-10-methyl-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV (CA4), obtained from bovine lung microsomes | Bioorg Med Chem Lett 12: 1551-7 (2002) BindingDB Entry DOI: 10.7270/Q2B56K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50114180 ((1R,2R,5R)-4-((1R,5S,8S,10R,11S,12S)-3,12-Dihydrox...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV (CA4), obtained from bovine lung microsomes | Bioorg Med Chem Lett 12: 1551-7 (2002) BindingDB Entry DOI: 10.7270/Q2B56K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11632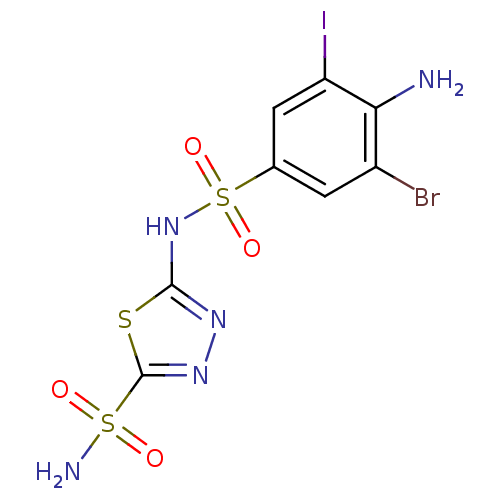 (2-N-(4-amino-3-bromo-5-iodobenzene)-1,3,4-thiadiaz...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14723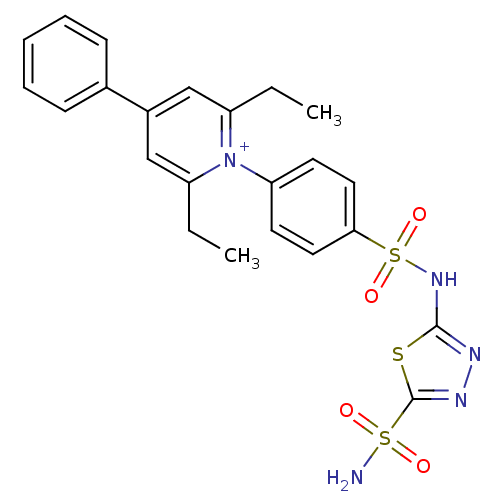 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11633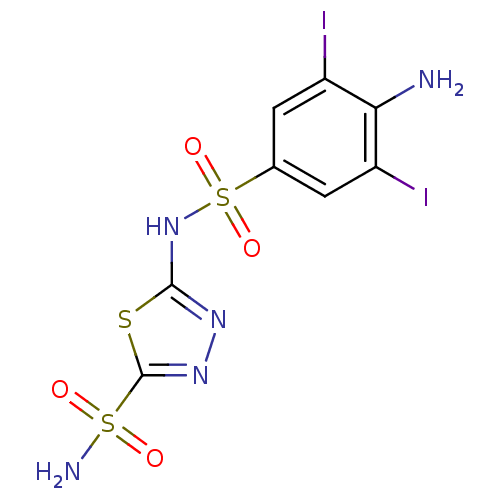 (2-N-(4-amino-3,5-diiodobenzene)-1,3,4-thiadiazole-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11623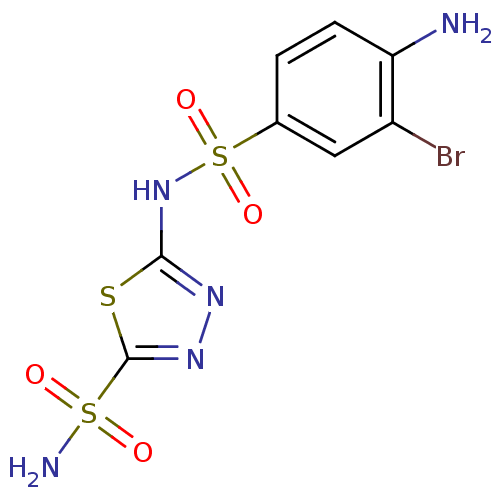 (2-N-(4-amino-3-bromobenzene)-1,3,4-thiadiazole-2,5...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14736 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11622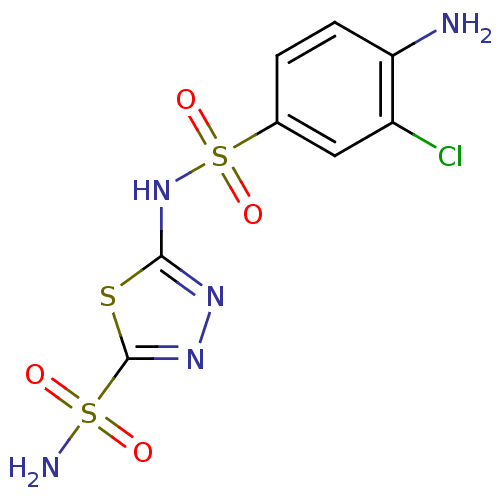 (β-CA inhibitor, 1 | 2-N-(4-amino-3-chlorobenz...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11621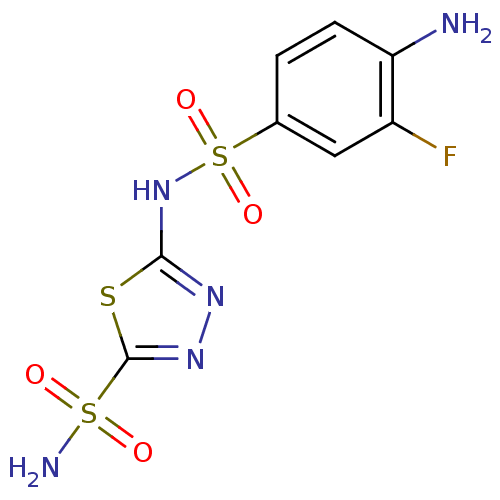 (2-N-(4-amino-3-fluorobenzene)-1,3,4-thiadiazole-2,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11624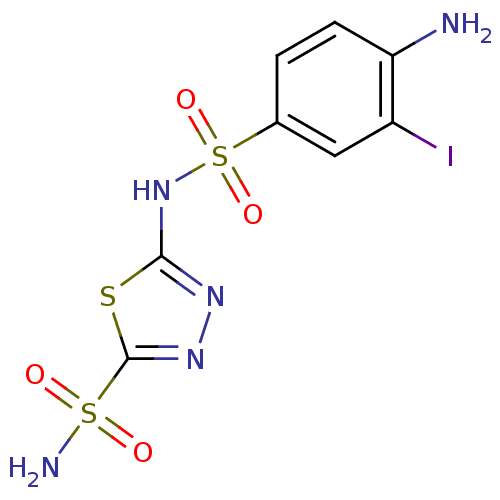 (2-N-(4-amino-3-iodobenzene)-1,3,4-thiadiazole-2,5-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11630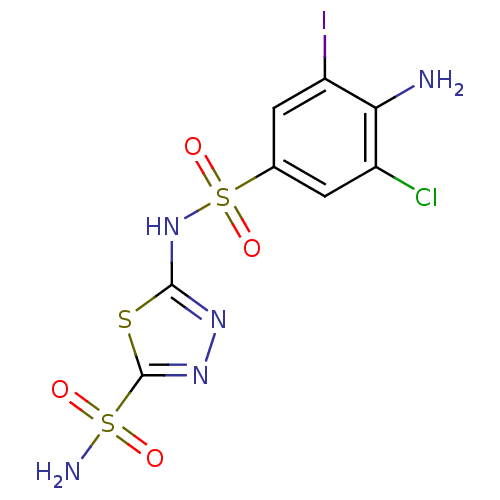 (2-N-(4-amino-3-chloro-5-iodobenzene)-1,3,4-thiadia...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11427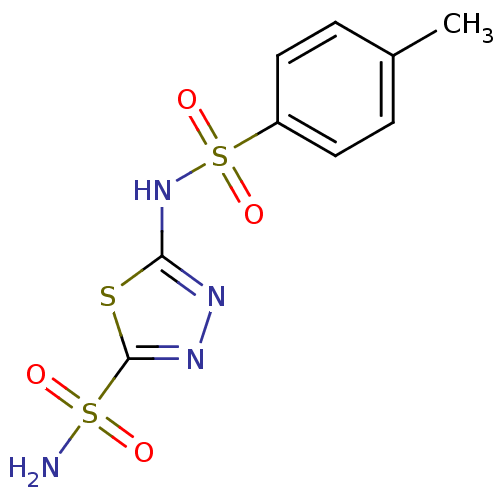 (2-N-(4-methylbenzene)-1,3,4-thiadiazole-2,5-disulf...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibitory effect on bovine Carbonic anhydrase IV | J Med Chem 45: 312-20 (2002) BindingDB Entry DOI: 10.7270/Q2ZP46TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14719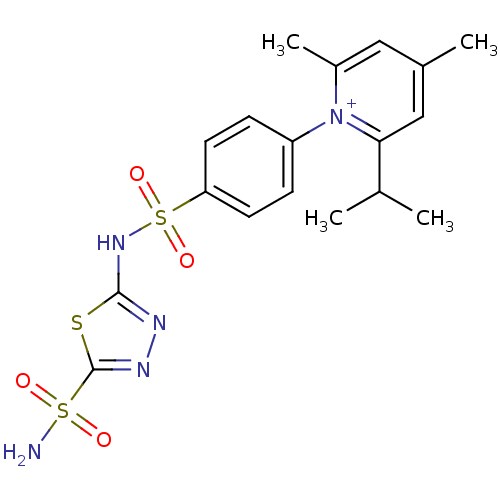 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14727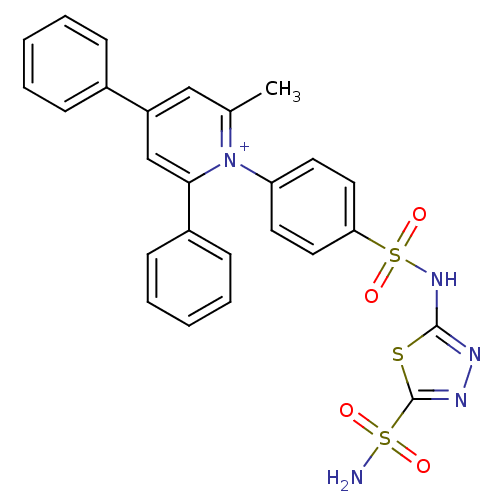 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14728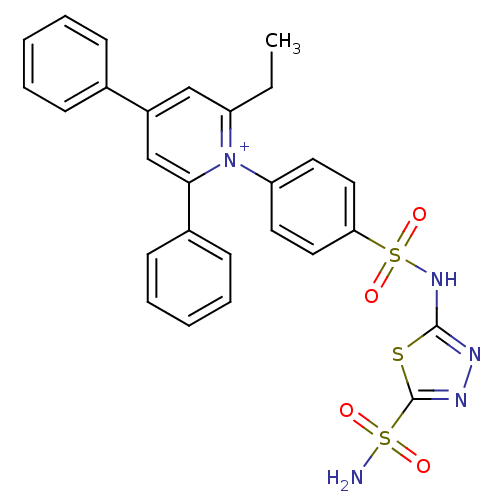 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50108585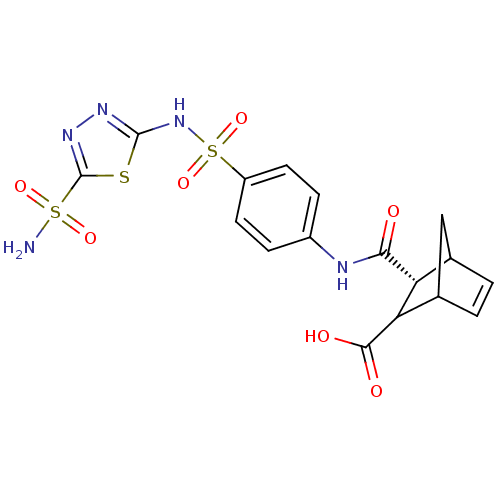 (3-[4-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylsulfamoyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibitory effect on bovine Carbonic anhydrase IV | J Med Chem 45: 312-20 (2002) BindingDB Entry DOI: 10.7270/Q2ZP46TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50292007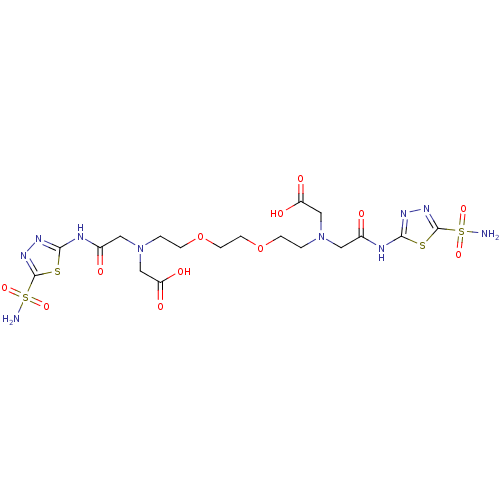 (CHEMBL1795064 | CHEMBL286828 | {{2-[2-(2-{Carboxym...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV | J Med Chem 45: 1466-76 (2002) BindingDB Entry DOI: 10.7270/Q2N29XN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11616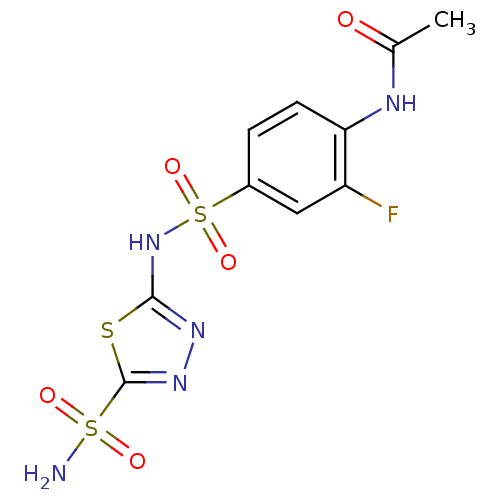 (5-(4-Acetamido-3-fluorobenzenesulfonamido)-1,3,4-t...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11618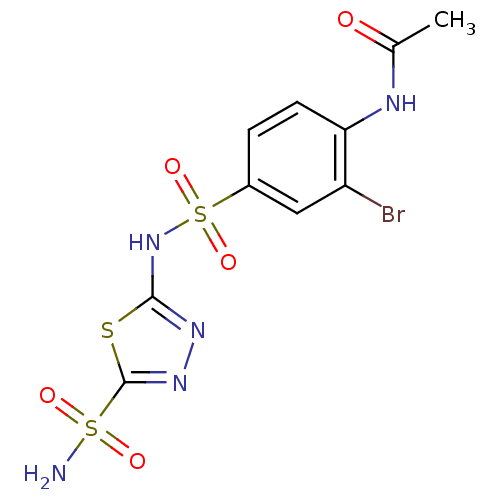 (5-(4-Acetamido-3-bromobenzenesulfonamido)-1,3,4-th...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11617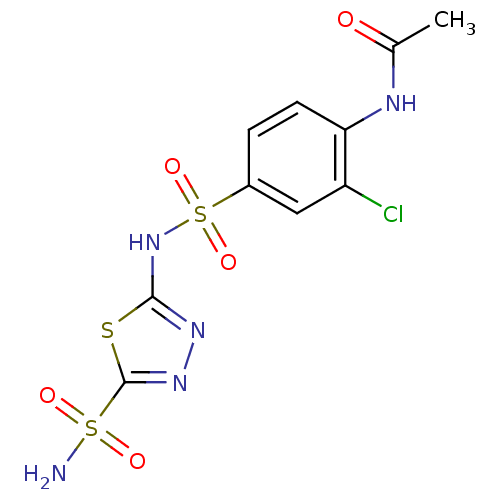 (5-(4-Acetamido-3-chlorobenzenesulfonamido)-1,3,4-t...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11616 (5-(4-Acetamido-3-fluorobenzenesulfonamido)-1,3,4-t...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 1272-9 (2004) Article DOI: 10.1021/jm031057+ BindingDB Entry DOI: 10.7270/Q2MW2FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50088762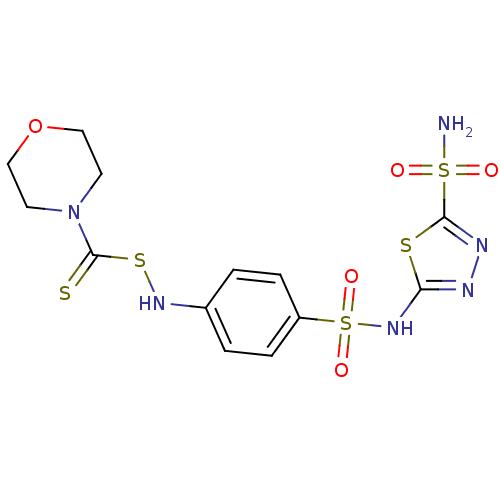 (CHEMBL13614 | N,N-dialkylthio-carbonylsulfenylamin...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibition of bovine carbonic anhydrase IV from bovine lung microsomes | Bioorg Med Chem Lett 10: 1117-20 (2000) BindingDB Entry DOI: 10.7270/Q2WW7J6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50087265 (5-{4-[(7-Chloro-2-oxo-2H-chromen-4-ylmethyl)-amino...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibition of bovine Carbonic anhydrase IV determined by esterase method | Bioorg Med Chem Lett 10: 673-6 (2000) BindingDB Entry DOI: 10.7270/Q2RV0P61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50114134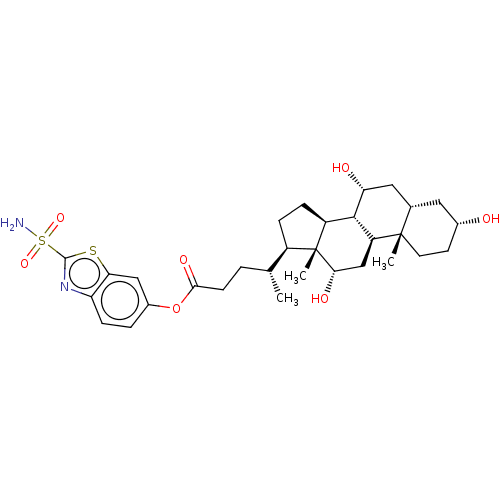 ((R)-4-((8S,10S,12S,14R,15R,17S)-3,12-Di(R)-hydroxy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV (CA4), obtained from bovine lung microsomes | Bioorg Med Chem Lett 12: 1551-7 (2002) BindingDB Entry DOI: 10.7270/Q2B56K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50097307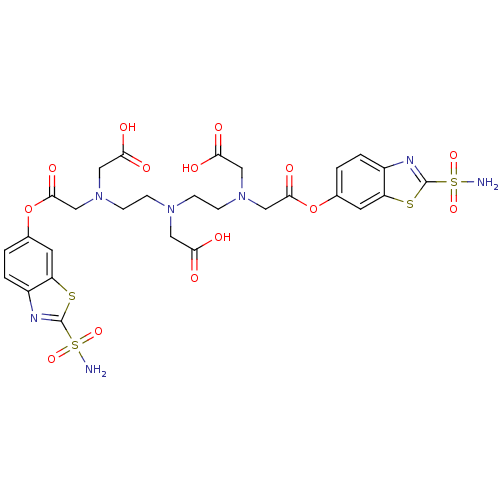 (CHEMBL408856 | [[2-(Carboxymethyl-{2-[carboxymethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV | J Med Chem 45: 1466-76 (2002) BindingDB Entry DOI: 10.7270/Q2N29XN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50292100 (CHEMBL284354 | {{2-[2-(2-{Carboxymethyl-[(2-sulfam...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV | J Med Chem 45: 1466-76 (2002) BindingDB Entry DOI: 10.7270/Q2N29XN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14726 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canadian Institutes of Health Research | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 2337-47 (2004) Article DOI: 10.1021/jm031079w BindingDB Entry DOI: 10.7270/Q2DV1H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50114115 ((R)-4-((8S,10S,12S,14R,15R,17S)-3,12-Di(R)-hydroxy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV (CA4), obtained from bovine lung microsomes | Bioorg Med Chem Lett 12: 1551-7 (2002) BindingDB Entry DOI: 10.7270/Q2B56K7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11617 (5-(4-Acetamido-3-chlorobenzenesulfonamido)-1,3,4-t...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1546 total ) | Next | Last >> |