

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM31592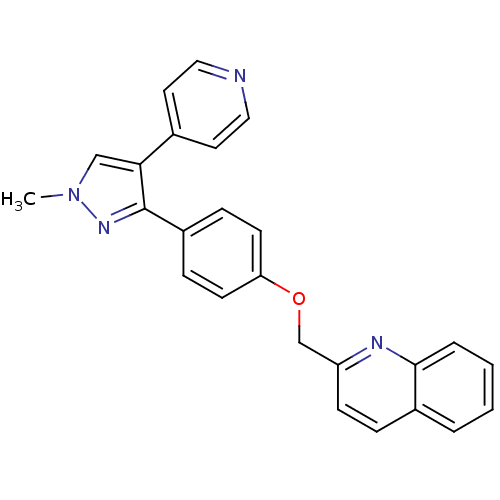 (PF-2545920 | US9138494, MP-10 | substituted pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-(6,7-dimethoxycinnolin-4-yl)-N-isopropyl-3-methylpyridin-2-amine from PDE10A in Sprague-Dawley rat striatum | J Med Chem 55: 4776-87 (2012) Article DOI: 10.1021/jm3002372 BindingDB Entry DOI: 10.7270/Q2WM1FFG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM50365964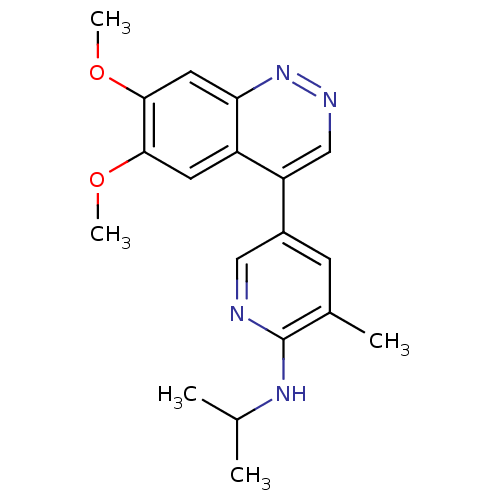 (CHEMBL1956235 | CHEMBL2070530) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]5-(6,7-dimethoxycinnolin-4-yl)-N-isopropyl-3-methylpyridin-2-amine from PDE10A in Sprague-Dawley rat striatum | J Med Chem 55: 4776-87 (2012) Article DOI: 10.1021/jm3002372 BindingDB Entry DOI: 10.7270/Q2WM1FFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14768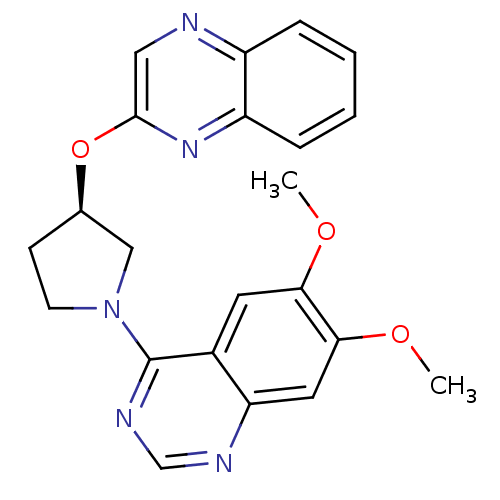 ((R)-6,7-Dimethoxy-4-[3-(quinoxalin-2-yloxy)-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14766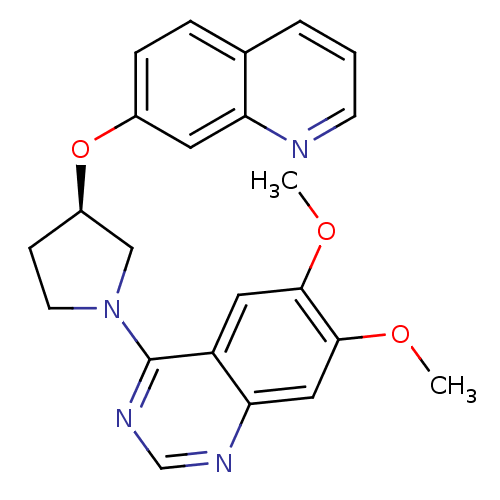 ((R)-6,7-Dimethoxy-4-[3-(quinolin-7-yloxy)-pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14764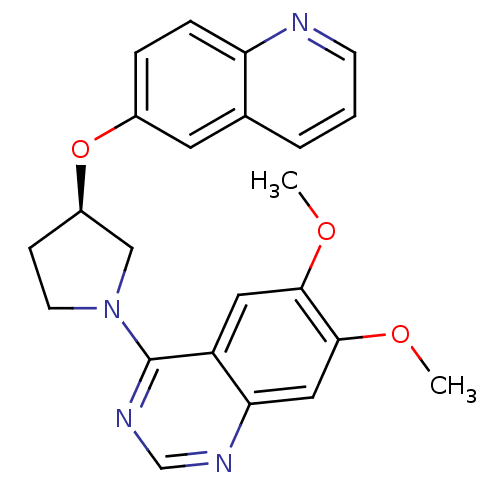 ((R)-6,7-Dimethoxy-4-[3-(quinolin-6-yloxy)-pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14760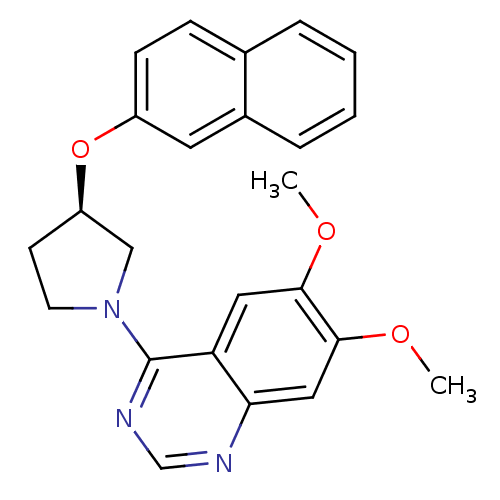 ((R)-6,7-Dimethoxy-4-[3-(naphthalen-2-yloxy)-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14754 (1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-isoq...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14763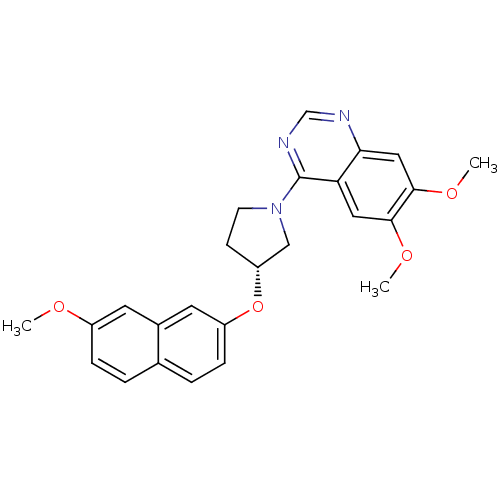 ((R)-6,7-Dimethoxy-4-[3-(7-methoxy-naphthalen-2-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14765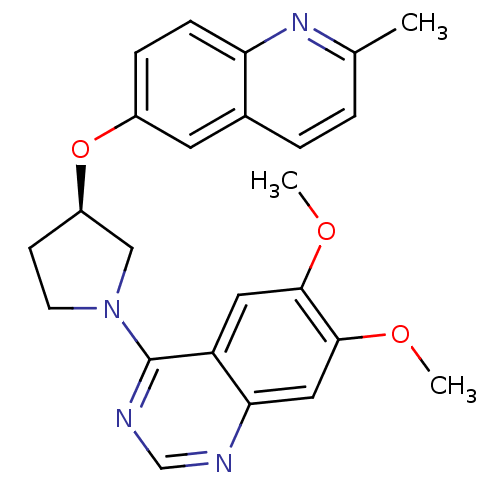 ((R)-6,7-Dimethoxy-4-[3-(2-methyl-quinolin-6-yloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14762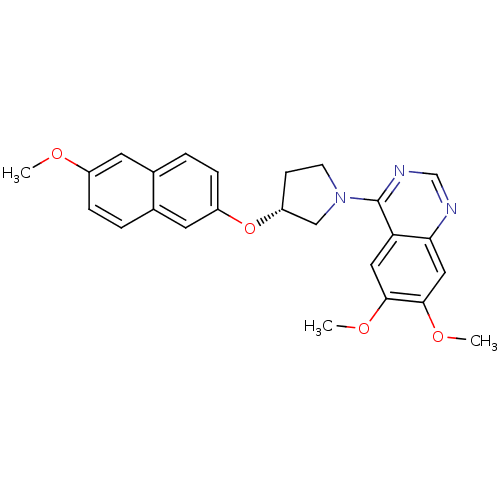 ((R)-6,7-Dimethoxy-4-[3-(6-methoxy-naphthalen-2-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14767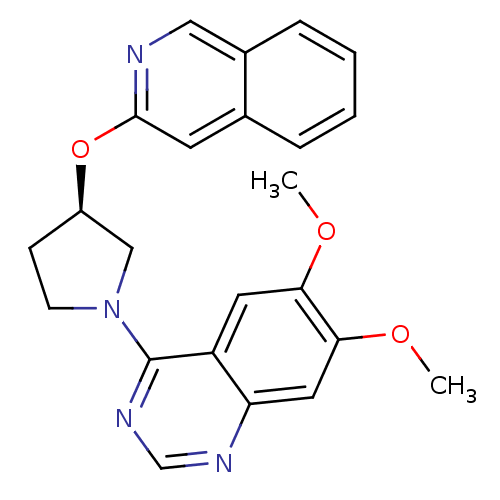 ((R)-4-[3-(Isoquinolin-3-yloxy)-pyrrolidin-1-yl]-6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14755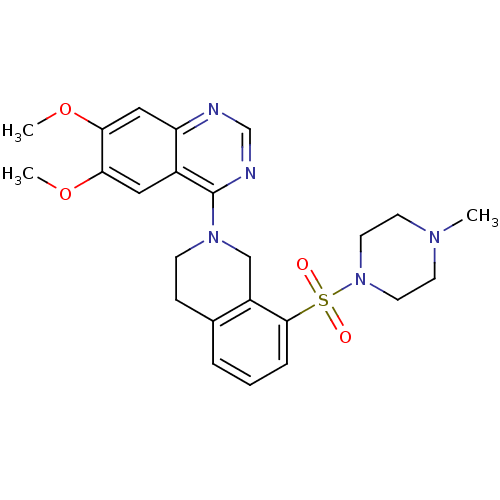 (6,7-dimethoxy-4-[8-(4-methylpiperazin-1-yl)sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | 25 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14756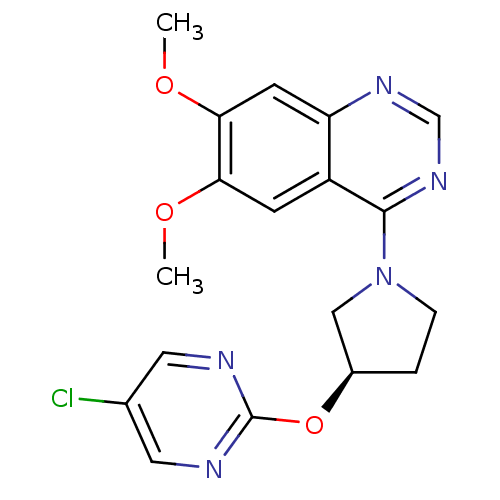 ((R)-4-[3-(5-Chloro-pyrimidin-2-yloxy)-pyrrolidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14748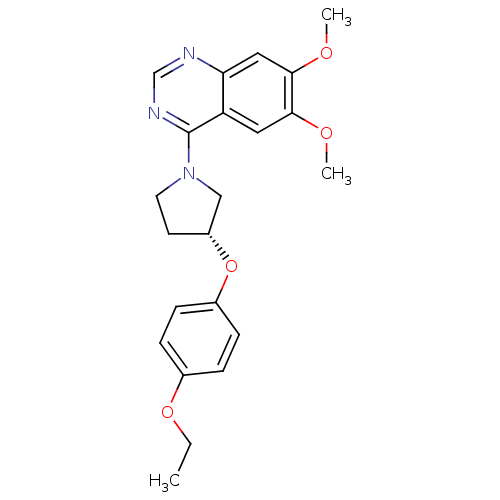 ((R)-4-[3-(4-Ethoxy-phenoxy)-pyrrolidin-1-yl]-6,7-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 54 | -41.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14745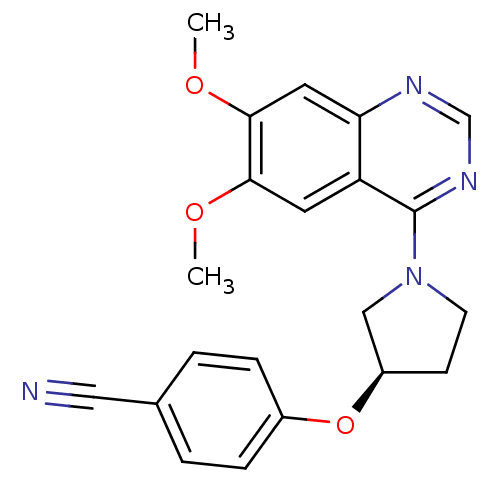 ((R)-4-[1-(6,7-Dimethoxy-quinazolin-4-yl)-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 56 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14753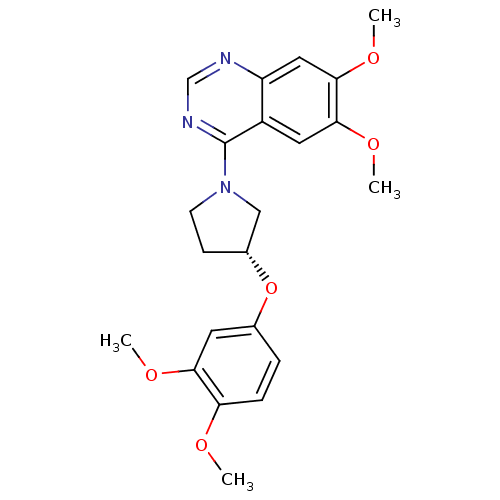 ((R)-4-[3-(3,4-Dimethoxy-phenoxy)-pyrrolidin-1-yl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 67 | -40.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14752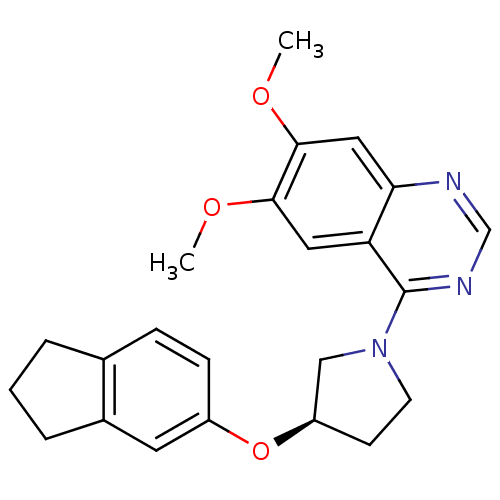 ((R)-4-[3-(Indan-5-yloxy)-pyrrolidin-1-yl]-6,7-dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 68 | -40.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14751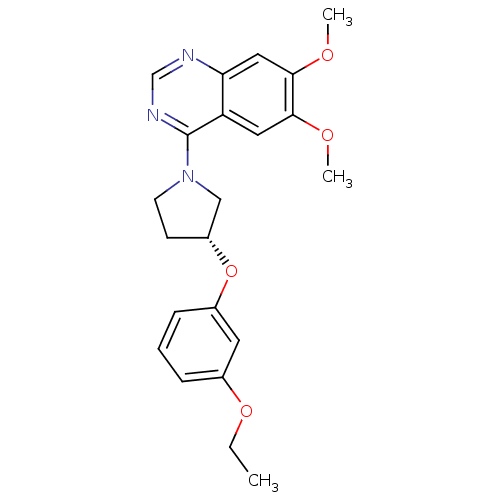 ((R)-4-[3-(3-Ethoxy-phenoxy)-pyrrolidin-1-yl]-6,7-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 94 | -39.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14747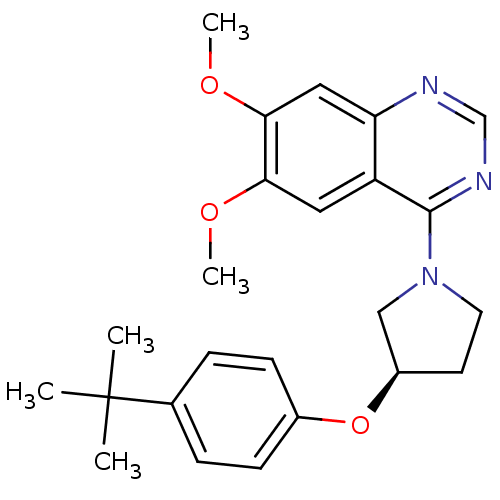 ((R)-4-[3-(4-tert-Butyl-phenoxy)-pyrrolidin-1-yl]-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | -39.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14759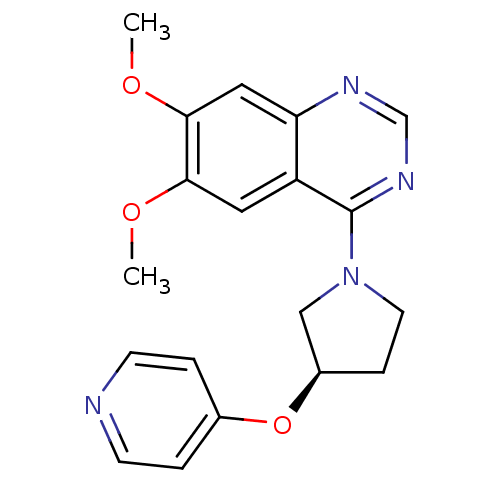 ((R)-6,7-Dimethoxy-4-[3-(pyridin-4-yloxy)-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14757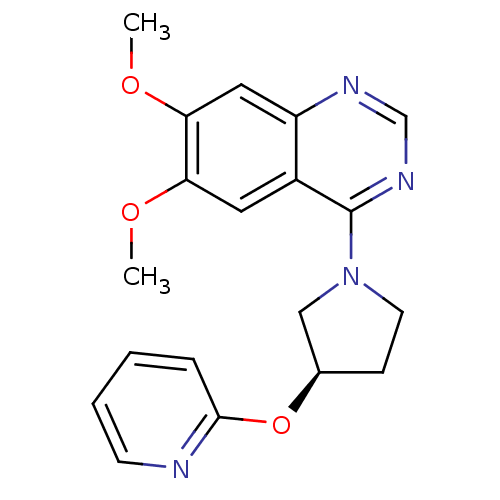 ((R)-6,7-Dimethoxy-4-[3-(pyridin-2-yloxy)-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14758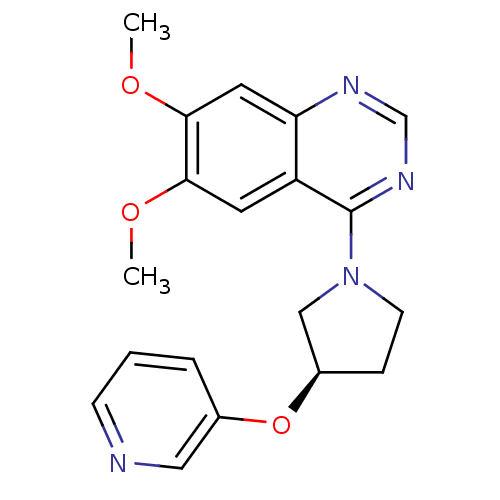 ((R)-6,7-Dimethoxy-4-[3-(pyridin-3-yloxy)-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14744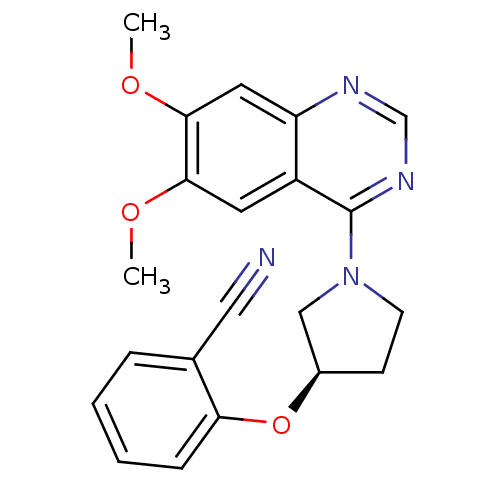 ((R)-2-[1-(6,7-Dimethoxy-quinazolin-4-yl)-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 130 | -38.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14750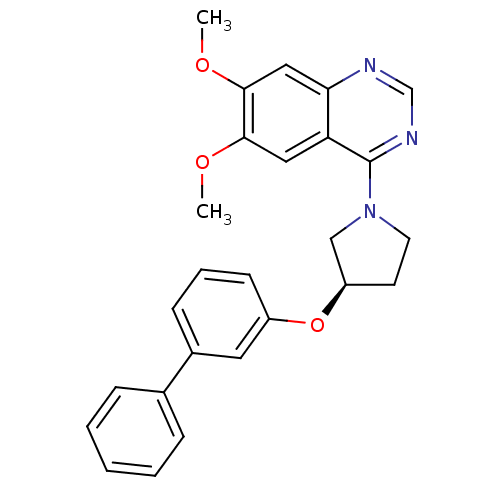 ((R)-4-[3-(Biphenyl-3-yloxy)-pyrrolidin-1-yl]-6,7-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 136 | -38.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14749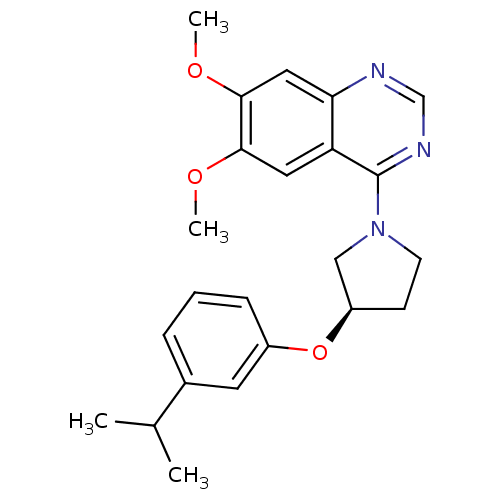 ((R)-4-[3-(3-Isopropyl-phenoxy)-pyrrolidin-1-yl]-6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 171 | -38.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14746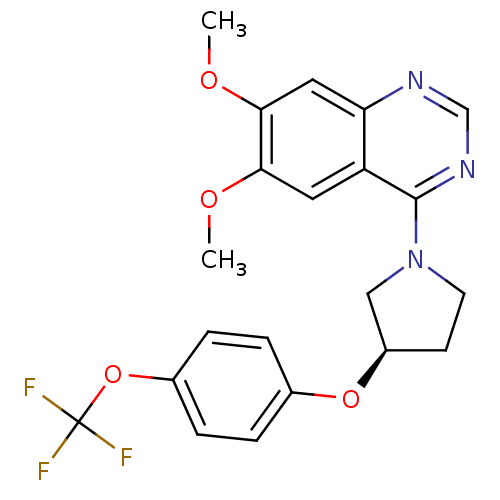 ((R)-6,7-Dimethoxy-4-[3-(4-trifluoromethoxy-phenoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 185 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14743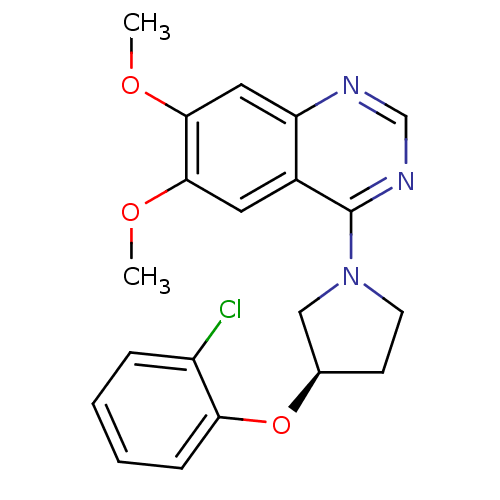 ((R)-4-[3-(2-Chloro-phenoxy)-pyrrolidin-1-yl]-6,7-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 198 | -37.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14761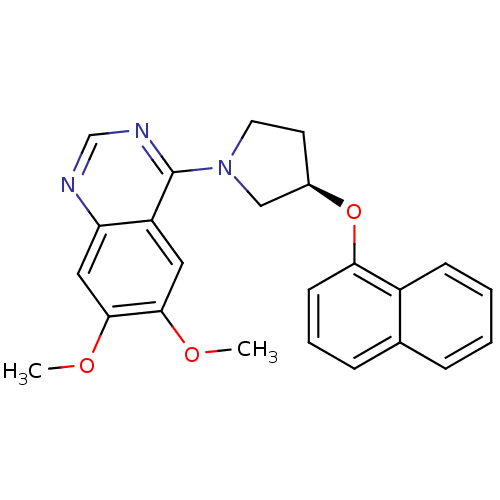 ((R)-6,7-Dimethoxy-4-[3-(naphthalen-1-yloxy)-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14741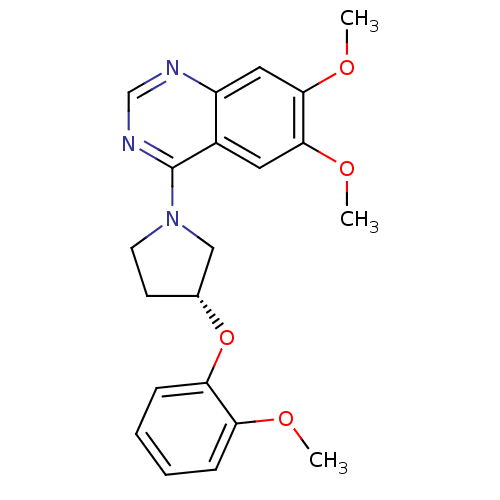 ((R)-6,7-Dimethoxy-4-[3-(2-methoxy-phenoxy)-pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 240 | -37.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14740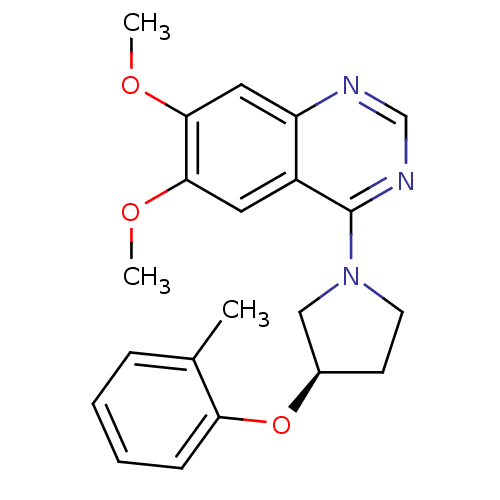 ((R)-6,7-Dimethoxy-4-(3-o-tolyloxy-pyrrolidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 326 | -36.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14742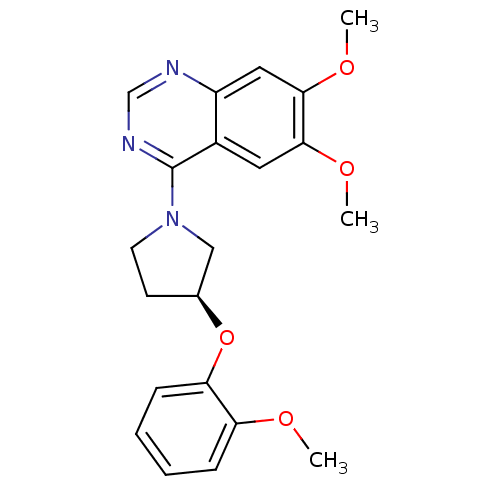 ((S)-6,7-Dimethoxy-4-[3-(2-methoxy-phenoxy)-pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 641 | -35.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM14739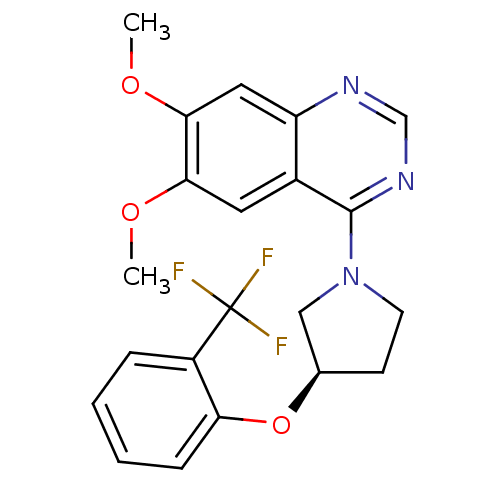 ((R)-6,7-Dimethoxy-4-[3-(2-trifluoromethyl-phenoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 811 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Pfizer | Assay Description PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using a scintillation proximity assay (SPA). [3H]-AMP was captured by... | J Med Chem 50: 182-5 (2007) Article DOI: 10.1021/jm060653b BindingDB Entry DOI: 10.7270/Q2930RDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM115431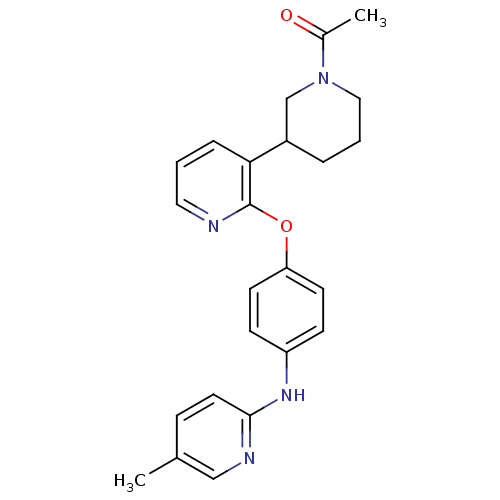 (US8637500, 301 | US8637500, 454 | US8637500, 455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 μL of serial diluted PDE10A (BPS Bi... | US Patent US8637500 (2014) BindingDB Entry DOI: 10.7270/Q2XP73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM115430 (US8637500, 299) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0450 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 μL of serial diluted PDE10A (BPS Bi... | US Patent US8637500 (2014) BindingDB Entry DOI: 10.7270/Q2XP73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM115427 (US8637500, 296) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 μL of serial diluted PDE10A (BPS Bi... | US Patent US8637500 (2014) BindingDB Entry DOI: 10.7270/Q2XP73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM115428 (US8637500, 297) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 μL of serial diluted PDE10A (BPS Bi... | US Patent US8637500 (2014) BindingDB Entry DOI: 10.7270/Q2XP73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM142557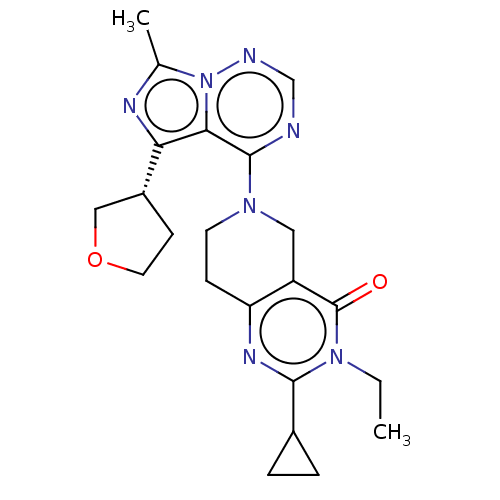 (US8933224, 150) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0697 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. US Patent | Assay Description The ability of a compound to inhibit PDE10 enzymatic activity can be demonstrated by any number of assays that are known in the art. The products of ... | US Patent US8933224 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM142550 (US8933224, 143 | US8933224, 144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0837 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. US Patent | Assay Description The ability of a compound to inhibit PDE10 enzymatic activity can be demonstrated by any number of assays that are known in the art. The products of ... | US Patent US8933224 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM142481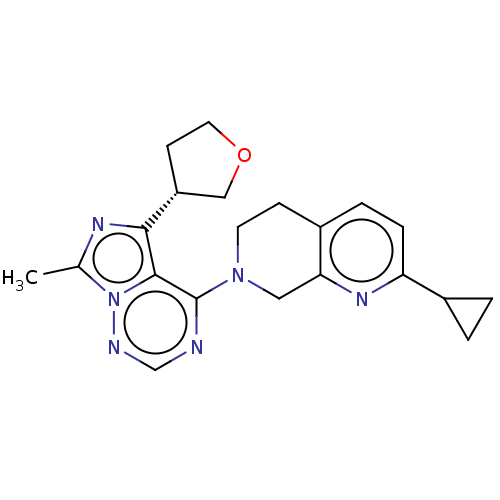 (US8933224, 74) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0896 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. US Patent | Assay Description The ability of a compound to inhibit PDE10 enzymatic activity can be demonstrated by any number of assays that are known in the art. The products of ... | US Patent US8933224 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM142544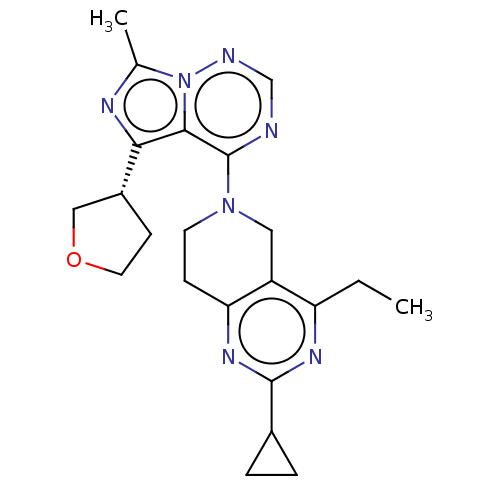 (US8933224, 137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0950 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. US Patent | Assay Description The ability of a compound to inhibit PDE10 enzymatic activity can be demonstrated by any number of assays that are known in the art. The products of ... | US Patent US8933224 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM115424 (US8637500, 293) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0980 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 μL of serial diluted PDE10A (BPS Bi... | US Patent US8637500 (2014) BindingDB Entry DOI: 10.7270/Q2XP73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM142536 (US8933224, 129) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0998 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. US Patent | Assay Description The ability of a compound to inhibit PDE10 enzymatic activity can be demonstrated by any number of assays that are known in the art. The products of ... | US Patent US8933224 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM142459 (US8933224, 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.101 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. US Patent | Assay Description The ability of a compound to inhibit PDE10 enzymatic activity can be demonstrated by any number of assays that are known in the art. The products of ... | US Patent US8933224 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM142451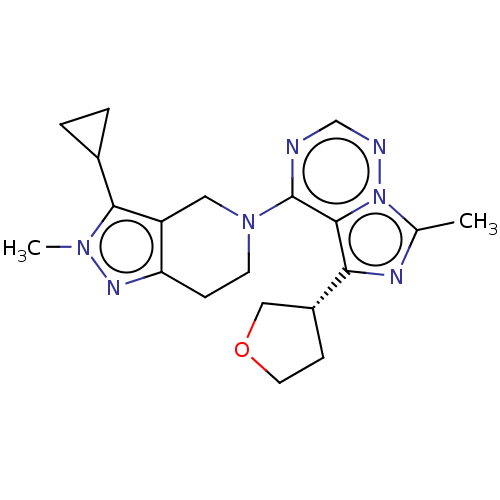 (US8933224, 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.102 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. US Patent | Assay Description The ability of a compound to inhibit PDE10 enzymatic activity can be demonstrated by any number of assays that are known in the art. The products of ... | US Patent US8933224 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM142550 (US8933224, 143 | US8933224, 144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.106 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. US Patent | Assay Description The ability of a compound to inhibit PDE10 enzymatic activity can be demonstrated by any number of assays that are known in the art. The products of ... | US Patent US8933224 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM142442 (US8933224, 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.123 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. US Patent | Assay Description The ability of a compound to inhibit PDE10 enzymatic activity can be demonstrated by any number of assays that are known in the art. The products of ... | US Patent US8933224 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM142555 (US8933224, 148) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.125 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. US Patent | Assay Description The ability of a compound to inhibit PDE10 enzymatic activity can be demonstrated by any number of assays that are known in the art. The products of ... | US Patent US8933224 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM142559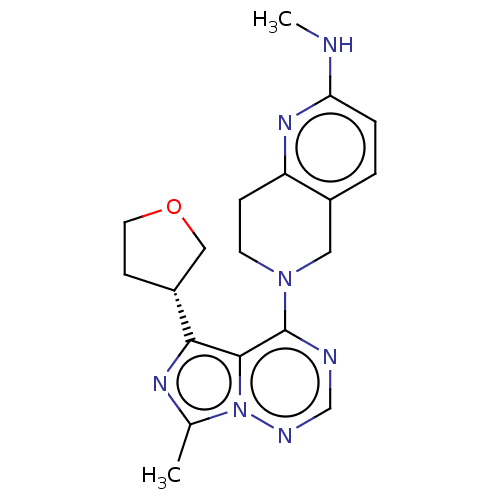 (US8933224, 152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. US Patent | Assay Description The ability of a compound to inhibit PDE10 enzymatic activity can be demonstrated by any number of assays that are known in the art. The products of ... | US Patent US8933224 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM142493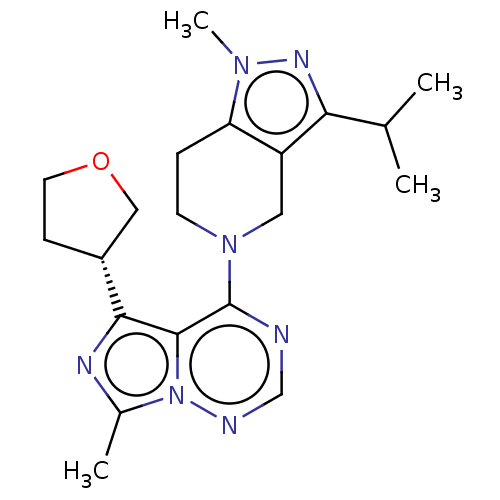 (US8933224, 86) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.132 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. US Patent | Assay Description The ability of a compound to inhibit PDE10 enzymatic activity can be demonstrated by any number of assays that are known in the art. The products of ... | US Patent US8933224 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM115425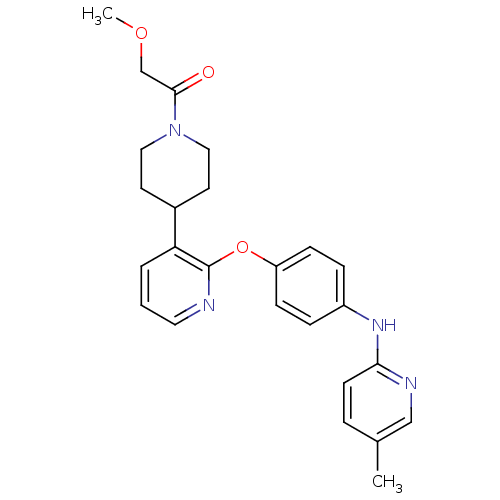 (US8637500, 294) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.132 | n/a | n/a | n/a | n/a | n/a | 25 |
Amgen Inc. US Patent | Assay Description An IMAP TR-FRET assay was used to analyze the enzyme activity (Molecular Devices Corp., Sunnyvale Calif.). 5 μL of serial diluted PDE10A (BPS Bi... | US Patent US8637500 (2014) BindingDB Entry DOI: 10.7270/Q2XP73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1444 total ) | Next | Last >> |