 Found 6 hits for monomerid = 50021437
Found 6 hits for monomerid = 50021437 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021437
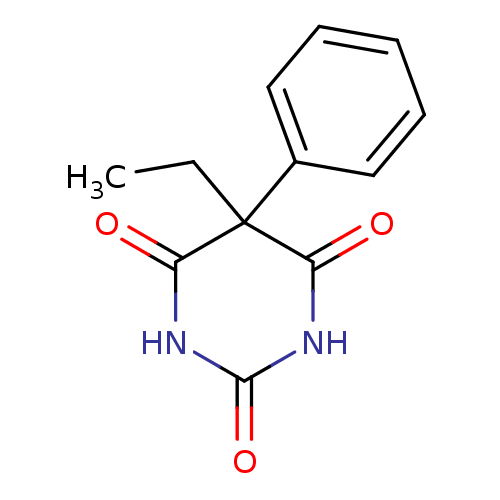
(5-Ethyl-5-phenylbarbituric acid | 5-Phenyl-5-ethyl...)Show InChI InChI=1S/C12H12N2O3/c1-2-12(8-6-4-3-5-7-8)9(15)13-11(17)14-10(12)16/h3-7H,2H2,1H3,(H2,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.02E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TCG Lifesciences Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Eur J Med Chem 46: 618-30 (2011)
Article DOI: 10.1016/j.ejmech.2010.11.042
BindingDB Entry DOI: 10.7270/Q2WQ052W |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021437

(5-Ethyl-5-phenylbarbituric acid | 5-Phenyl-5-ethyl...)Show InChI InChI=1S/C12H12N2O3/c1-2-12(8-6-4-3-5-7-8)9(15)13-11(17)14-10(12)16/h3-7H,2H2,1H3,(H2,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against IKr potassium channel |
Bioorg Med Chem Lett 14: 4771-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.070
BindingDB Entry DOI: 10.7270/Q208661K |
More data for this
Ligand-Target Pair | |
Sodium channel protein type I I alpha subunit
(Rattus norvegicus) | BDBM50021437

(5-Ethyl-5-phenylbarbituric acid | 5-Phenyl-5-ethyl...)Show InChI InChI=1S/C12H12N2O3/c1-2-12(8-6-4-3-5-7-8)9(15)13-11(17)14-10(12)16/h3-7H,2H2,1H3,(H2,13,14,15,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Howard University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]BTX-B binding to neurotoxin site 2 of sodium channel of rat cerebral cortex synaptoneurosomes |
J Med Chem 38: 4033-43 (1995)
Article DOI: 10.1021/jm00020a019
BindingDB Entry DOI: 10.7270/Q2DV1NM8 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type I I alpha subunit
(Homo sapiens (Human)) | BDBM50021437

(5-Ethyl-5-phenylbarbituric acid | 5-Phenyl-5-ethyl...)Show InChI InChI=1S/C12H12N2O3/c1-2-12(8-6-4-3-5-7-8)9(15)13-11(17)14-10(12)16/h3-7H,2H2,1H3,(H2,13,14,15,16,17) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-BTX binding to guinea pig voltage-dependent sodium channel |
J Med Chem 29: 1512-6 (1986)
BindingDB Entry DOI: 10.7270/Q29C6WF2 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM50021437

(5-Ethyl-5-phenylbarbituric acid | 5-Phenyl-5-ethyl...)Show InChI InChI=1S/C12H12N2O3/c1-2-12(8-6-4-3-5-7-8)9(15)13-11(17)14-10(12)16/h3-7H,2H2,1H3,(H2,13,14,15,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human BSEP expressed in fall armyworm sf9 cell plasma membrane vesicles assessed as reduction in vesicle-associated [3H]-taurocholate t... |
Toxicol Sci 118: 485-500 (2010)
Article DOI: 10.1093/toxsci/kfq269
BindingDB Entry DOI: 10.7270/Q26Q20JN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021437

(5-Ethyl-5-phenylbarbituric acid | 5-Phenyl-5-ethyl...)Show InChI InChI=1S/C12H12N2O3/c1-2-12(8-6-4-3-5-7-8)9(15)13-11(17)14-10(12)16/h3-7H,2H2,1H3,(H2,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.02E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel in HEK293 cells by voltage-clamp method |
Eur J Med Chem 43: 2479-88 (2008)
Article DOI: 10.1016/j.ejmech.2007.12.025
BindingDB Entry DOI: 10.7270/Q2542PTB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data