

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM107261 US8598345, 203::US8598345, Table 1-48/2-7
SMILES: O=C(Nc1ccccn1)c1cc(Oc2cncnc2)ccn1
InChI Key: InChIKey=FELLWSPKJLIKKD-UHFFFAOYSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM107261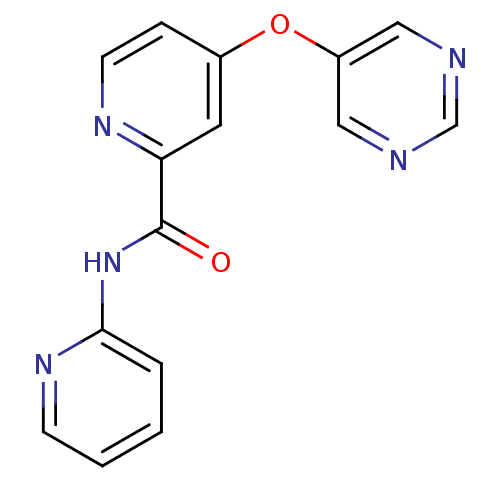 (US8598345, 203 | US8598345, Table 1-48/2-7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University US Patent | Assay Description mGluR5 activity was determined in a cell-based assay. | US Patent US8598345 (2013) BindingDB Entry DOI: 10.7270/Q2RR1WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor (Rattus norvegicus (Rat)) | BDBM107261 (US8598345, 203 | US8598345, Table 1-48/2-7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University US Patent | Assay Description mGluR5 activity was determined in a cell-based assay. | US Patent US8598345 (2013) BindingDB Entry DOI: 10.7270/Q2RR1WX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||