

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM107751 CHEMBL2089063::US8598217, 89
SMILES: CN(C)C1CCN(CC1)c1ccc2nc([nH]c2c1)C(=O)c1ccnc(c1)-c1cncc2ccccc12
InChI Key: InChIKey=POJGZHZSQVZDGP-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107751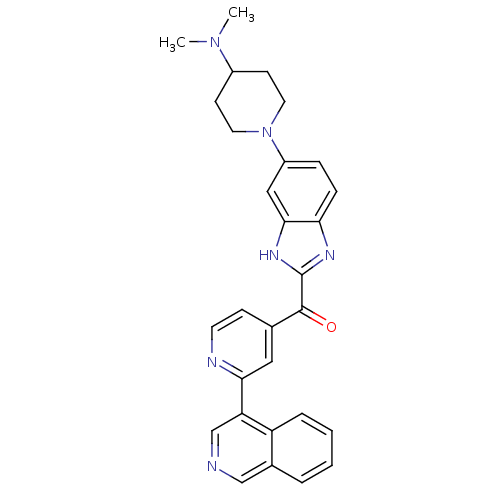 (CHEMBL2089063 | US8598217, 89) | UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM107751 (CHEMBL2089063 | US8598217, 89) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CDK1/cyclin B assessed as inhibition of Tamra-H1 phosphorylation by IMAP-FP assay | ACS Med Chem Lett 3: 445-449 (2012) Article DOI: 10.1021/ml200241a BindingDB Entry DOI: 10.7270/Q2P270DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM107751 (CHEMBL2089063 | US8598217, 89) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CDK6/cyclin D3 assessed as inhibition of pRb Ser780 phosphorylation after 120 mins by TR-FRET assay | ACS Med Chem Lett 3: 445-449 (2012) Article DOI: 10.1021/ml200241a BindingDB Entry DOI: 10.7270/Q2P270DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||