

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCc1nc2c(C)cc(C)nc2n1Cc1ccc(\C=C\CN2CCN(CC2)C(C)C)cc1
InChI Key: InChIKey=UAMIBPKKKLTAKG-BQYQJAHWSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123515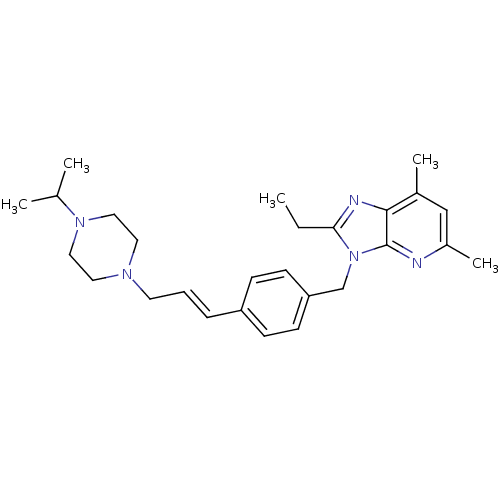 (US8748435, 40) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Novartis AG US Patent | Assay Description Serial dilutions of compounds (stock in 10 mM DMSO) are prepared by first diluting the compounds in DMSO followed by a 1:50 dilution into assay buffe... | US Patent US8748435 (2014) BindingDB Entry DOI: 10.7270/Q2F18XDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Mus musculus) | BDBM123515 (US8748435, 40) | UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse GPR4 expressed in HEK cells assessed as inhibition of pH-induced cAMP accumulation preincubated for 15 mins followed by ... | Bioorg Med Chem 25: 4512-4525 (2017) Article DOI: 10.1016/j.bmc.2017.06.050 BindingDB Entry DOI: 10.7270/Q2NS0XB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123515 (US8748435, 40) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in African green monkey COS7 cells assessed as inhibition of pH 6.5-induced cAMP accumulation after 30 mi... | Bioorg Med Chem 25: 4512-4525 (2017) Article DOI: 10.1016/j.bmc.2017.06.050 BindingDB Entry DOI: 10.7270/Q2NS0XB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM123515 (US8748435, 40) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor (unknown origin) | Bioorg Med Chem 25: 4512-4525 (2017) Article DOI: 10.1016/j.bmc.2017.06.050 BindingDB Entry DOI: 10.7270/Q2NS0XB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM123515 (US8748435, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from recombinant human ERG expressed in HEK293 cells | Bioorg Med Chem 25: 4512-4525 (2017) Article DOI: 10.1016/j.bmc.2017.06.050 BindingDB Entry DOI: 10.7270/Q2NS0XB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Homo sapiens (Human)) | BDBM123515 (US8748435, 40) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins by HTRF assay | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GSC2 (Saccharomyces cerevisiae) | BDBM123515 (US8748435, 40) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from recombinant human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM123515 (US8748435, 40) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-R(-)-alpha-Methyl[imidazole-2.5(n)]histamine from human recombinant histamine H3 receptor expressed in CHOK1 cell membranes afte... | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (Rattus norvegicus) | BDBM123515 (US8748435, 40) | UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at rat GPR4 expressed in HEK cells assessed as inhibition of pH-induced cAMP accumulation preincubated for 15 mins followed by cA... | Bioorg Med Chem 25: 4512-4525 (2017) Article DOI: 10.1016/j.bmc.2017.06.050 BindingDB Entry DOI: 10.7270/Q2NS0XB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||