

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cc1cc(I)c(O)c(c1)C(=O)Nc1ccc(C(N)=N)c(F)c1
InChI Key: InChIKey=GDPHNLIBJUAUNC-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14158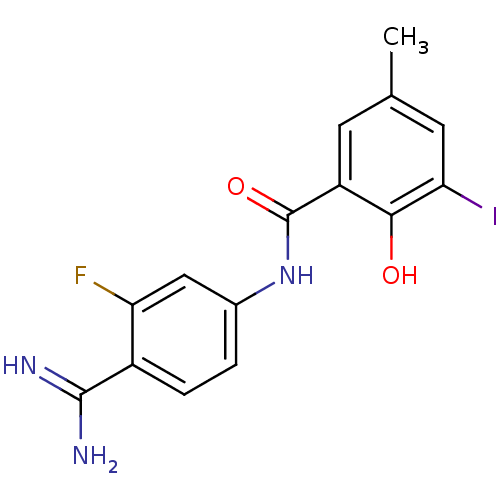 (APC-11922 | CHEMBL293260 | N-(4-carbamimidoyl-3-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 12: 2023-6 (2002) BindingDB Entry DOI: 10.7270/Q2ZG6RK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14158 (APC-11922 | CHEMBL293260 | N-(4-carbamimidoyl-3-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | -8.86 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14158 (APC-11922 | CHEMBL293260 | N-(4-carbamimidoyl-3-fl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14158 (APC-11922 | CHEMBL293260 | N-(4-carbamimidoyl-3-fl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 12: 2023-6 (2002) BindingDB Entry DOI: 10.7270/Q2ZG6RK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14158 (APC-11922 | CHEMBL293260 | N-(4-carbamimidoyl-3-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14158 (APC-11922 | CHEMBL293260 | N-(4-carbamimidoyl-3-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of coagulation factor X | Bioorg Med Chem Lett 12: 2023-6 (2002) BindingDB Entry DOI: 10.7270/Q2ZG6RK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM14158 (APC-11922 | CHEMBL293260 | N-(4-carbamimidoyl-3-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM14158 (APC-11922 | CHEMBL293260 | N-(4-carbamimidoyl-3-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Activation of plasminogen | Bioorg Med Chem Lett 12: 2023-6 (2002) BindingDB Entry DOI: 10.7270/Q2ZG6RK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14158 (APC-11922 | CHEMBL293260 | N-(4-carbamimidoyl-3-fl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM14158 (APC-11922 | CHEMBL293260 | N-(4-carbamimidoyl-3-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of plasmin | Bioorg Med Chem Lett 12: 2023-6 (2002) BindingDB Entry DOI: 10.7270/Q2ZG6RK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM14158 (APC-11922 | CHEMBL293260 | N-(4-carbamimidoyl-3-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||