

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM150758 2-(2-Hydroxyphenyl)-4H-chromen-4-one (3d)::US9271961, 2'-Hydroxyflavone
SMILES: Oc1ccccc1-c1cc(=O)c2ccccc2o1
InChI Key: InChIKey=ZZLQHXCRRMUGQJ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM150758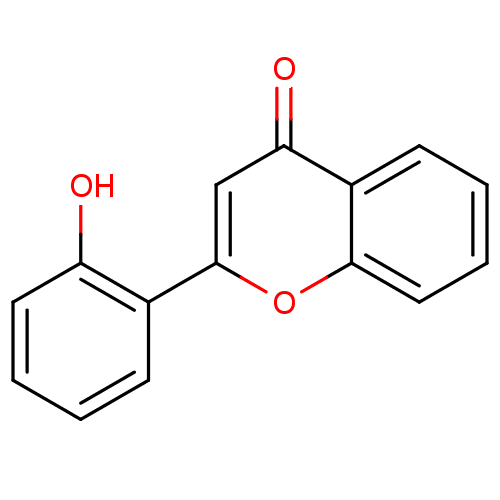 (2-(2-Hydroxyphenyl)-4H-chromen-4-one (3d) | US9271...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM150758 (2-(2-Hydroxyphenyl)-4H-chromen-4-one (3d) | US9271...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | Assay Description The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =... | Bioorg Chem 58: 72-80 (2015) Article DOI: 10.1016/j.bioorg.2014.11.008 BindingDB Entry DOI: 10.7270/Q2PG1QFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM150758 (2-(2-Hydroxyphenyl)-4H-chromen-4-one (3d) | US9271...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Homo sapiens (Human)) | BDBM150758 (2-(2-Hydroxyphenyl)-4H-chromen-4-one (3d) | US9271...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Antagonist activity at AR in human LNCAP cells assessed as reduction in DHT-induced cell growth treated for every 2 days for 4 days by hemocytometry | Eur J Med Chem 157: 1143-1152 (2018) Article DOI: 10.1016/j.ejmech.2018.08.069 BindingDB Entry DOI: 10.7270/Q24X5BHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Homo sapiens (Human)) | BDBM150758 (2-(2-Hydroxyphenyl)-4H-chromen-4-one (3d) | US9271...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Antagonist activity at AR in human LNCAP cells assessed as reduction in DHT-induced transcriptional activation after 24 hrs by luciferase reporter ge... | Eur J Med Chem 157: 1143-1152 (2018) Article DOI: 10.1016/j.ejmech.2018.08.069 BindingDB Entry DOI: 10.7270/Q24X5BHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-Hydroxysteroid Dehydrogenase 5 (17-beta-HSD5, AKR1C3) (Homo sapiens (Human)) | BDBM150758 (2-(2-Hydroxyphenyl)-4H-chromen-4-one (3d) | US9271...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM150758 (2-(2-Hydroxyphenyl)-4H-chromen-4-one (3d) | US9271...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||