

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: C[C@H]1[C@@H]2N(CC3CC3)CC[C@@]1(C)c1cc(NC(CO)CO)ccc1C2=O
InChI Key: InChIKey=DZAXUMWVIDHSHQ-JBZDTAIVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM168150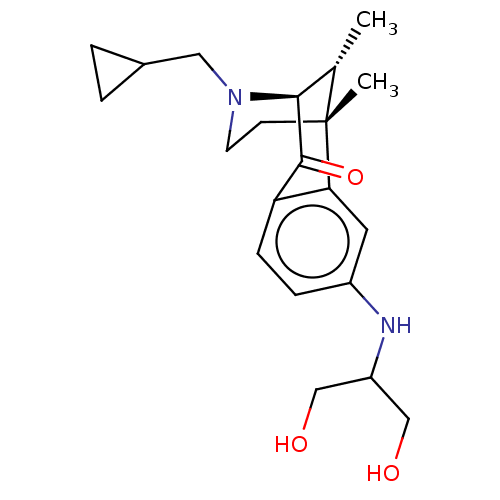 (US10081602, Example 24 | US10865186, Compound 24 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 494 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Nektar Therapeutics US Patent | Assay Description The binding affinities of certain compounds of the present invention were evaluated using radioligand binding assays in membranes prepared from CHO-K... | US Patent US10081602 (2018) BindingDB Entry DOI: 10.7270/Q2N87CT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM168150 (US10081602, Example 24 | US10865186, Compound 24 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 494 | -8.60 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Nektar Therapeutics US Patent | Assay Description Competition binding experiments were conducted by incubating membrane protein to equilibrium in triplicate in the presence of a fixed concentration o... | US Patent US9688638 (2017) BindingDB Entry DOI: 10.7270/Q2VM49FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM168150 (US10081602, Example 24 | US10865186, Compound 24 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 494 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nektar Therapeutics US Patent | Assay Description The binding affinities of certain compounds of the present invention were evaluated using radioligand binding assays in membranes prepared from CHO-K... | US Patent US10865186 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM168150 (US10081602, Example 24 | US10865186, Compound 24 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nektar Therapeutics US Patent | US Patent US10865186 (2020) | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM168150 (US10081602, Example 24 | US10865186, Compound 24 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.81E+3 | -7.83 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Nektar Therapeutics US Patent | Assay Description Competition binding experiments were conducted by incubating membrane protein to equilibrium in triplicate in the presence of a fixed concentration o... | US Patent US9688638 (2017) BindingDB Entry DOI: 10.7270/Q2VM49FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM168150 (US10081602, Example 24 | US10865186, Compound 24 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Nektar Therapeutics US Patent | Assay Description The binding affinities of certain compounds of the present invention were evaluated using radioligand binding assays in membranes prepared from CHO-K... | US Patent US10081602 (2018) BindingDB Entry DOI: 10.7270/Q2N87CT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM168150 (US10081602, Example 24 | US10865186, Compound 24 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 598 | n/a | n/a | n/a | n/a |
Nektar Therapeutics US Patent | Assay Description Inhibition of cAMP accumulation by select compounds was measured in forskolin-stimulated CHO-K1 cells stably expressing KOR. CHO-K1 cells stably expr... | US Patent US10081602 (2018) BindingDB Entry DOI: 10.7270/Q2N87CT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM168150 (US10081602, Example 24 | US10865186, Compound 24 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 107 | n/a | n/a | n/a | n/a |
Nektar Therapeutics US Patent | Assay Description Inhibition of cAMP accumulation by select compounds was measured in forskolin-stimulated CHO-K1 cells stably expressing KOR. CHO-K1 cells stably expr... | US Patent US9688638 (2017) BindingDB Entry DOI: 10.7270/Q2VM49FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM168150 (US10081602, Example 24 | US10865186, Compound 24 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 580 | n/a | n/a | n/a | n/a |
Nektar Therapeutics US Patent | Assay Description Inhibition of cAMP accumulation by select compounds was measured in forskolin-stimulated CHO-K1 cells stably expressing KOR. CHO-K1 cells stably expr... | US Patent US9688638 (2017) BindingDB Entry DOI: 10.7270/Q2VM49FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM168150 (US10081602, Example 24 | US10865186, Compound 24 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 107 | n/a | n/a | n/a | n/a |
Nektar Therapeutics US Patent | Assay Description Inhibition of cAMP accumulation by select compounds was measured in forskolin-stimulated CHO-K1 cells stably expressing KOR. CHO-K1 cells stably expr... | US Patent US10081602 (2018) BindingDB Entry DOI: 10.7270/Q2N87CT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||