

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM175398 1,3-Dimethyl-8-[4-methoxy-3-{2-(piperidin-1-yl)-2-oxoethoxy}-phenyl]xanthine (16d, RB-417)
SMILES: COc1ccc(cc1OCC(=O)N1CCCCC1)-c1nc2n(C)c(=O)n(C)c(=O)c2[nH]1
InChI Key: InChIKey=BMBWRZPUMJHDJI-UHFFFAOYSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM175398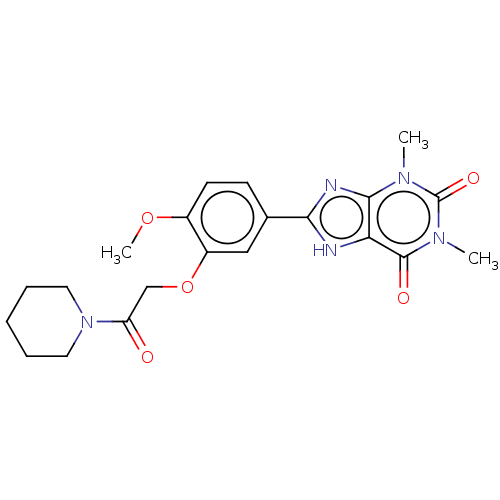 (1,3-Dimethyl-8-[4-methoxy-3-{2-(piperidin-1-yl)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University | Assay Description Dissociation constants at A1, A2A and A3 receptors (Ki-values) were determined in radioligand competition experiments as reported earlier [19,20]. Al... | Bioorg Chem 65: 26-37 (2016) Article DOI: 10.1016/j.bioorg.2016.01.003 BindingDB Entry DOI: 10.7270/Q2C53JN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM175398 (1,3-Dimethyl-8-[4-methoxy-3-{2-(piperidin-1-yl)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University | Assay Description Dissociation constants at A1, A2A and A3 receptors (Ki-values) were determined in radioligand competition experiments as reported earlier [19,20]. Al... | Bioorg Chem 65: 26-37 (2016) Article DOI: 10.1016/j.bioorg.2016.01.003 BindingDB Entry DOI: 10.7270/Q2C53JN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (A3) (Homo sapiens (Human)) | BDBM175398 (1,3-Dimethyl-8-[4-methoxy-3-{2-(piperidin-1-yl)-2-...) | GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University | Assay Description Dissociation constants at A1, A2A and A3 receptors (Ki-values) were determined in radioligand competition experiments as reported earlier [19,20]. Al... | Bioorg Chem 65: 26-37 (2016) Article DOI: 10.1016/j.bioorg.2016.01.003 BindingDB Entry DOI: 10.7270/Q2C53JN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM175398 (1,3-Dimethyl-8-[4-methoxy-3-{2-(piperidin-1-yl)-2-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Panjab University | Assay Description Dissociation constants at A1, A2A and A3 receptors (Ki-values) were determined in radioligand competition experiments as reported earlier [19,20]. Al... | Bioorg Chem 65: 26-37 (2016) Article DOI: 10.1016/j.bioorg.2016.01.003 BindingDB Entry DOI: 10.7270/Q2C53JN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||