

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM176272 US10047103, 305::US9688695, 305
SMILES: COc1nn2cc(nc2s1)-c1cc2c(OCc3csc(n3)-c3ccc(cc3)C(=O)NCC(F)(F)F)cc(OC)cc2o1
InChI Key: InChIKey=KOQWYAURZBRJBF-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteinase-activated receptor 4 (PAR4) (Homo sapiens (Human)) | BDBM176272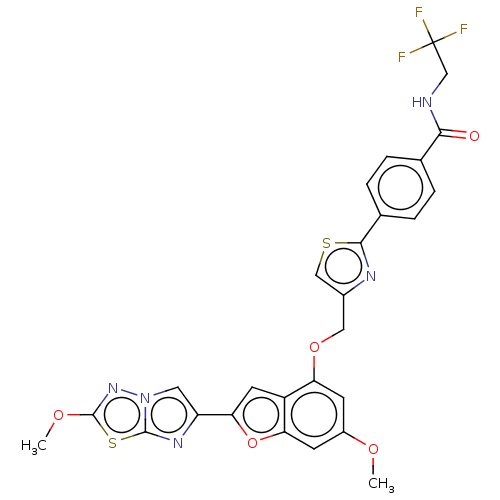 (US10047103, 305 | US9688695, 305) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | 25 |
BRISTOL-MYERS SQUIBB COMPANY US Patent | Assay Description Briefly, HEK293 EBNA PAR4 clone 20664.1J cells were plated 24 hrs. prior to experiment in 384 well, Poly-D-Lysine coated, black, clear bottom plates ... | US Patent US9688695 (2017) BindingDB Entry DOI: 10.7270/Q28K777N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176272 (US10047103, 305 | US9688695, 305) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (PAR4) (Homo sapiens (Human)) | BDBM176272 (US10047103, 305 | US9688695, 305) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (PAR4) (Homo sapiens (Human)) | BDBM176272 (US10047103, 305 | US9688695, 305) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | 25 |
BRISTOL-MYERS SQUIBB COMPANY US Patent | Assay Description Briefly, PRP or washed platelet suspension (100 μl) was pre-incubated for 5 minutes at room temperature with varying concentrations of compounds... | US Patent US9688695 (2017) BindingDB Entry DOI: 10.7270/Q28K777N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||