

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM179875 US10231963, Table B.21::US10752592, Compound TABLE B.3::US9133125, Table B, Compound 3::US9656961, Example 00138
SMILES: NC(=O)c1ccc2C[C@@H]3[C@@H]4CCC(=C)C[C@]4(CCN3CC3CCC3)c2c1O
InChI Key: InChIKey=QTCGTXSVMPVICZ-YYDVJCTNSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179875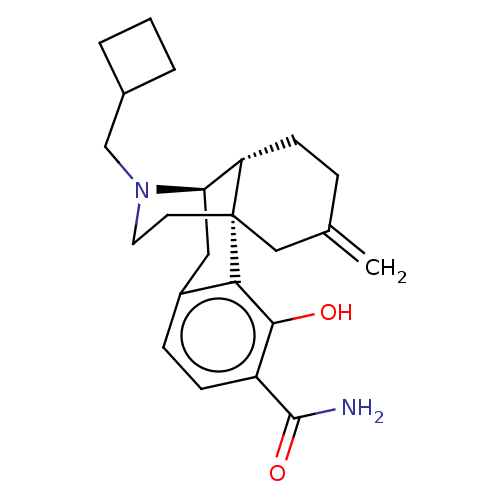 (US10231963, Table B.21 | US10752592, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | -13.7 | 1.30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for u opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal o... | US Patent US9133125 (2015) BindingDB Entry DOI: 10.7270/Q2736PPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179875 (US10231963, Table B.21 | US10752592, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179875 (US10231963, Table B.21 | US10752592, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10752592 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179875 (US10231963, Table B.21 | US10752592, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179875 (US10231963, Table B.21 | US10752592, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179875 (US10231963, Table B.21 | US10752592, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10752592 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179875 (US10231963, Table B.21 | US10752592, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179875 (US10231963, Table B.21 | US10752592, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179875 (US10231963, Table B.21 | US10752592, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179875 (US10231963, Table B.21 | US10752592, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10752592 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179875 (US10231963, Table B.21 | US10752592, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 0.530 | n/a | n/a | 7.4 | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The EC50 and Imax for μ opioid receptors was determined using a [I35S]GTPγS binding assay. This assay measures the functional properties of a compo... | US Patent US9133125 (2015) BindingDB Entry DOI: 10.7270/Q2736PPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||