

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC1(CC1)c1nc(-c2ccc(NC(=O)Nc3cc(F)cc(c3)C(F)(F)F)c3ccccc23)c2c(N)nccn12
InChI Key: InChIKey=ZSWNJFKGOATENB-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192736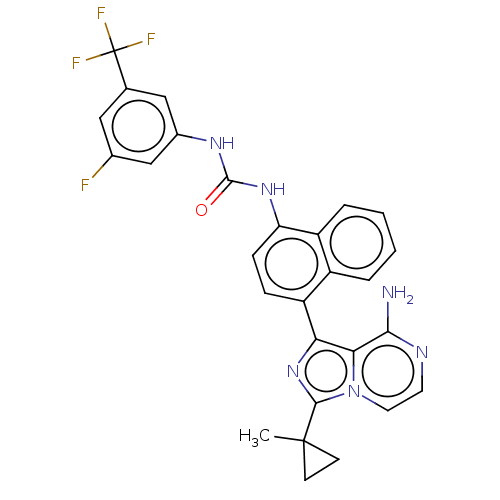 (KIRA analog, 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192736 (KIRA analog, 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||