

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM217379 CDDO-Me, 2
SMILES: COC(=O)[C@]12CCC(C)(C)C[C@H]1[C@H]1C(=O)C=C3[C@@]4(C)C=C(C#N)C(=O)C(C)(C)[C@@H]4CC[C@@]3(C)[C@]1(C)CC2
InChI Key: InChIKey=WPTTVJLTNAWYAO-KPOXMGGZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ghrelin O-acyltransferase (Homo sapiens (Human)) | BDBM217379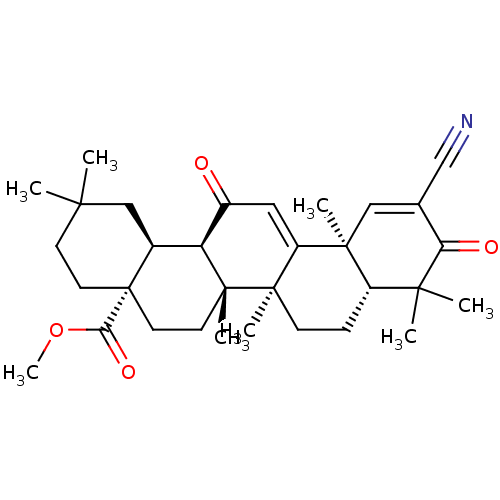 (CDDO-Me, 2) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Syracuse University | Assay Description Assays were performed with ~100 μg of membrane protein, as determined by a Bradford assay. The membrane fraction was preincubated with 1 μM met... | Biochemistry 56: 919-931 (2017) Article DOI: 10.1021/acs.biochem.6b01008 BindingDB Entry DOI: 10.7270/Q270808D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM217379 (CDDO-Me, 2) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Crystal Pharmatech Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of K+ stimulated gastric ATPase | J Med Chem 60: 8847-8857 (2017) Article DOI: 10.1021/acs.jmedchem.7b00971 BindingDB Entry DOI: 10.7270/Q2QV3PWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM217379 (CDDO-Me, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IFN-gamma stimulated STAT3 (unknown origin) expressed in human HeLa cells after 6 hrs by luciferase reporter gene assay | J Nat Prod 80: 2276-2283 (2017) Article DOI: 10.1021/acs.jnatprod.7b00271 BindingDB Entry DOI: 10.7270/Q2VX0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM217379 (CDDO-Me, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Progenra, Inc. Curated by ChEMBL | Assay Description Inhibition of USP7 (unknown origin) using human N-terminal GST-tagged UBA52 as substrate preincubated for 10 mins followed by substrate addition meas... | J Med Chem 61: 422-443 (2018) Article DOI: 10.1021/acs.jmedchem.7b00498 BindingDB Entry DOI: 10.7270/Q2CR5WVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor erythroid 2-related factor 2 (Homo sapiens (Human)) | BDBM217379 (CDDO-Me, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Activation of Nrf2 (unknown origin) expressed in human HaCaT-ARE-luc cells after 6 hrs by luciferase reporter gene assay | J Nat Prod 80: 2276-2283 (2017) Article DOI: 10.1021/acs.jnatprod.7b00271 BindingDB Entry DOI: 10.7270/Q2VX0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 2 (Homo sapiens (Human)) | BDBM217379 (CDDO-Me, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Progenra, Inc. Curated by ChEMBL | Assay Description Inhibition of USP2 (unknown origin) | J Med Chem 61: 422-443 (2018) Article DOI: 10.1021/acs.jmedchem.7b00498 BindingDB Entry DOI: 10.7270/Q2CR5WVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||