

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM221686 US10568884, Cpd 7::US9314464, 7::US9593100, Compound 7
SMILES: CCCS(=O)(=O)Nc1ccc(F)c(-c2nn(cc2-c2ccnc(NC[C@H](C)NC(=O)OC)n2)C(C)C)c1F
InChI Key: InChIKey=BEPGTMTXHVYWRS-HNNXBMFYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM221686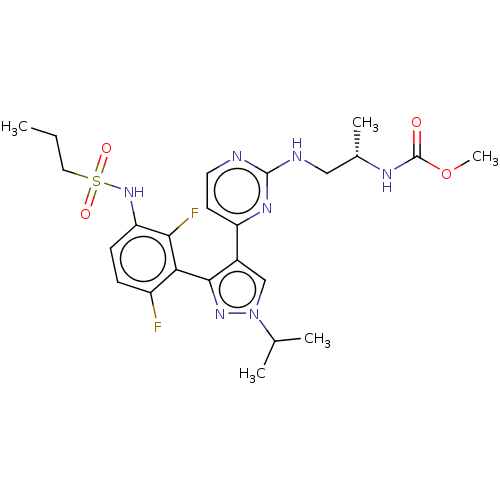 (US10568884, Cpd 7 | US9314464, 7 | US9593100, Comp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description B-Raf (V600E; 4 μM) and biotinylated Mek (kinase dead; 10 nM) were combined at 2× final concentrations in assay buffer (50 mM Tris, pH 7.5, ... | US Patent US9314464 (2016) BindingDB Entry DOI: 10.7270/Q2J67FS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-RAF V600E (Homo sapiens (Human)) | BDBM221686 (US10568884, Cpd 7 | US9314464, 7 | US9593100, Comp...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description The B-Raf kinase activity reaction was started by the addition of 10 μl piper well of 2×ATP (10 μM) diluted in assay buffer. After 3 hours,... | US Patent US10568884 (2020) BindingDB Entry DOI: 10.7270/Q2XK8HZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM221686 (US10568884, Cpd 7 | US9314464, 7 | US9593100, Comp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc. US Patent | Assay Description B-Raf (V600E; 4 pM) and biotinylated Mek (kinase dead; 10 nM) were combined at 2× final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgC... | US Patent US9593099 (2017) BindingDB Entry DOI: 10.7270/Q2BV7JPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM221686 (US10568884, Cpd 7 | US9314464, 7 | US9593100, Comp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc. US Patent | Assay Description B-Raf (V600E; 4 pM) and biotinylated Mek (kinase dead; 10 nM) were combined at 2× final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgC... | US Patent US9593100 (2017) BindingDB Entry DOI: 10.7270/Q2765HDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||