

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1ccc(cc1-c1cncnc1)C(=O)Nc1ccc(OC(F)(F)F)cc1
InChI Key: InChIKey=DPMHDCFAIUFGEP-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM222494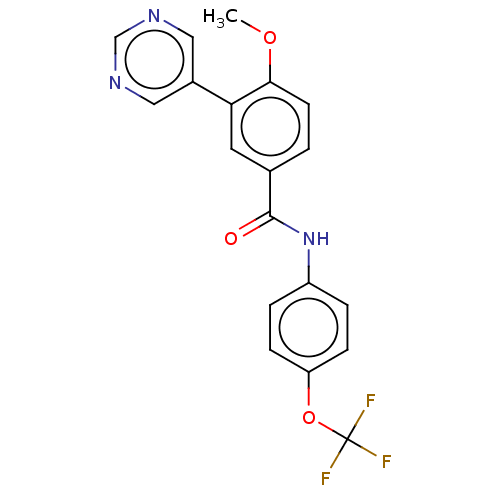 (US9315489, 30) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description For determination of ABL kinase activity, the radiometric filter-binding assay was used. The assay was performed by mixing 10 μL of the compound p... | US Patent US9315489 (2016) BindingDB Entry DOI: 10.7270/Q2H130WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GSC2 (Saccharomyces cerevisiae) | BDBM222494 (US9315489, 30) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by high throughput assay | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM222494 (US9315489, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m... | J Med Chem 61: 8120-8135 (2018) Article DOI: 10.1021/acs.jmedchem.8b01040 BindingDB Entry DOI: 10.7270/Q2FX7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||