 Found 7 hits for monomerid = 228129
Found 7 hits for monomerid = 228129 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nuclear receptor coactivator 2 [740-753]
() | BDBM228129
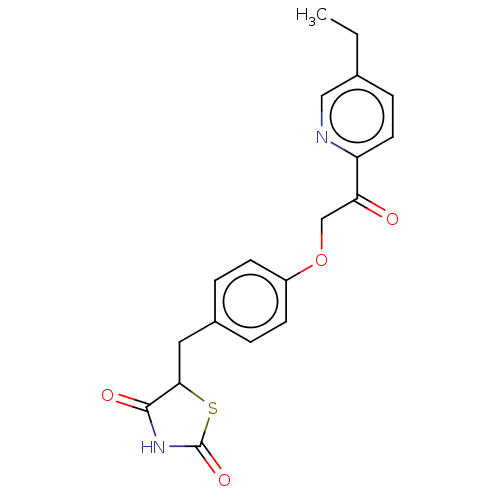
(US9562012, mitoglitazone)Show SMILES CCc1ccc(nc1)C(=O)COc1ccc(CC2SC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H18N2O4S/c1-2-12-5-8-15(20-10-12)16(22)11-25-14-6-3-13(4-7-14)9-17-18(23)21-19(24)26-17/h3-8,10,17H,2,9,11H2,1H3,(H,21,23,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| US Patent
| n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabolic Solutions Development Company, LLC
US Patent
| Assay Description
PPARγ binding is measured by a TR-FRET competitive binding assay using Invitrogen LanthaScreen TR-FRET PPARγ Competitive Binding Assay (I... |
US Patent US9562012 (2017)
BindingDB Entry DOI: 10.7270/Q26H4KDK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM228129

(US9562012, mitoglitazone)Show SMILES CCc1ccc(nc1)C(=O)COc1ccc(CC2SC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H18N2O4S/c1-2-12-5-8-15(20-10-12)16(22)11-25-14-6-3-13(4-7-14)9-17-18(23)21-19(24)26-17/h3-8,10,17H,2,9,11H2,1H3,(H,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SPTanis PharmaChem Consulting LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem 26: 5870-5884 (2018)
Article DOI: 10.1016/j.bmc.2018.10.033
BindingDB Entry DOI: 10.7270/Q2XD14DJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM228129

(US9562012, mitoglitazone)Show SMILES CCc1ccc(nc1)C(=O)COc1ccc(CC2SC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H18N2O4S/c1-2-12-5-8-15(20-10-12)16(22)11-25-14-6-3-13(4-7-14)9-17-18(23)21-19(24)26-17/h3-8,10,17H,2,9,11H2,1H3,(H,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SPTanis PharmaChem Consulting LLC
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem 26: 5870-5884 (2018)
Article DOI: 10.1016/j.bmc.2018.10.033
BindingDB Entry DOI: 10.7270/Q2XD14DJ |
More data for this
Ligand-Target Pair | |
Mitochondrial pyruvate carrier 2
(Homo sapiens) | BDBM228129

(US9562012, mitoglitazone)Show SMILES CCc1ccc(nc1)C(=O)COc1ccc(CC2SC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H18N2O4S/c1-2-12-5-8-15(20-10-12)16(22)11-25-14-6-3-13(4-7-14)9-17-18(23)21-19(24)26-17/h3-8,10,17H,2,9,11H2,1H3,(H,21,23,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SPTanis PharmaChem Consulting LLC
Curated by ChEMBL
| Assay Description
Displacement of photoprobe from MPC2 (unknown origin) |
Bioorg Med Chem 26: 5870-5884 (2018)
Article DOI: 10.1016/j.bmc.2018.10.033
BindingDB Entry DOI: 10.7270/Q2XD14DJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM228129

(US9562012, mitoglitazone)Show SMILES CCc1ccc(nc1)C(=O)COc1ccc(CC2SC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H18N2O4S/c1-2-12-5-8-15(20-10-12)16(22)11-25-14-6-3-13(4-7-14)9-17-18(23)21-19(24)26-17/h3-8,10,17H,2,9,11H2,1H3,(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SPTanis PharmaChem Consulting LLC
Curated by ChEMBL
| Assay Description
Competitive displacement of fluorescently labelled PPAR tracer ligand from GST-tagged human PPARgamma ligand binding domain after 1 hr in dark by TR-... |
Bioorg Med Chem 26: 5870-5884 (2018)
Article DOI: 10.1016/j.bmc.2018.10.033
BindingDB Entry DOI: 10.7270/Q2XD14DJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM228129

(US9562012, mitoglitazone)Show SMILES CCc1ccc(nc1)C(=O)COc1ccc(CC2SC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H18N2O4S/c1-2-12-5-8-15(20-10-12)16(22)11-25-14-6-3-13(4-7-14)9-17-18(23)21-19(24)26-17/h3-8,10,17H,2,9,11H2,1H3,(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 657 | n/a | n/a | n/a | n/a |
SPTanis PharmaChem Consulting LLC
Curated by ChEMBL
| Assay Description
Transactivation of GAL4-DBD fused human PPARgamma ligand binding domain expressed in UAS-bla HEL 293H cells preincubated for 16 hrs followed by FRET ... |
Bioorg Med Chem 26: 5870-5884 (2018)
Article DOI: 10.1016/j.bmc.2018.10.033
BindingDB Entry DOI: 10.7270/Q2XD14DJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
1,3-beta-glucan synthase component GSC2
(Saccharomyces cerevisiae) | BDBM228129

(US9562012, mitoglitazone)Show SMILES CCc1ccc(nc1)C(=O)COc1ccc(CC2SC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H18N2O4S/c1-2-12-5-8-15(20-10-12)16(22)11-25-14-6-3-13(4-7-14)9-17-18(23)21-19(24)26-17/h3-8,10,17H,2,9,11H2,1H3,(H,21,23,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SPTanis PharmaChem Consulting LLC
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem 26: 5870-5884 (2018)
Article DOI: 10.1016/j.bmc.2018.10.033
BindingDB Entry DOI: 10.7270/Q2XD14DJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data