

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM23209 (1R,2R,5S,8R,9R,10R,13R,14R,17S,18R,19R)-17-hydroxy-18-(hydroxymethyl)-1,2,14,18-tetramethyl-8-(prop-1-en-2-yl)pentacyclo[11.8.0.0^{2,10}.0^{5,9}.0^{14,19}]henicosane-5-carboxylic acid::23-Hydroxybetulinic Acid, 25
SMILES: [H][C@]12[C@@H](CC[C@@]1(CC[C@]1(C)[C@]2([H])CC[C@]2([H])[C@@]3(C)CC[C@H](O)[C@@](C)(CO)[C@]3([H])CC[C@@]12C)C(O)=O)C(C)=C
InChI Key: InChIKey=HXWLKAXCQLXHML-WGOZWDAUSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM23209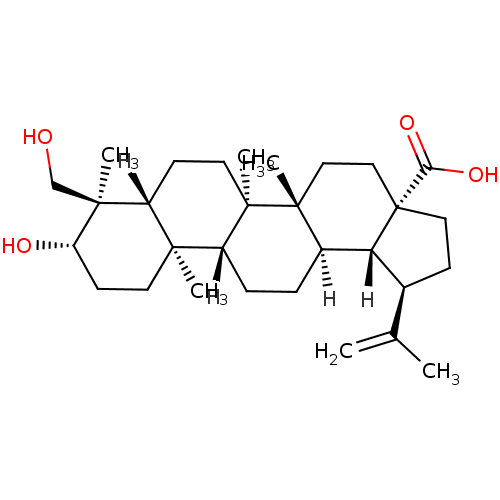 ((1R,2R,5S,8R,9R,10R,13R,14R,17S,18R,19R)-17-hydrox...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
China Pharmaceutical University | Assay Description The activity of the compounds is determined by measuring the inhibitory effect of the compounds in the direction of glycogen synthesis, the conversio... | J Med Chem 51: 3540-54 (2008) Article DOI: 10.1021/jm8000949 BindingDB Entry DOI: 10.7270/Q2WQ0233 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM23209 ((1R,2R,5S,8R,9R,10R,13R,14R,17S,18R,19R)-17-hydrox...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase A assessed as release of phosphate from glucose-1-phosphate after 25 mins | Bioorg Med Chem Lett 19: 6966-9 (2009) Article DOI: 10.1016/j.bmcl.2009.10.055 BindingDB Entry DOI: 10.7270/Q28G8MNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM23209 ((1R,2R,5S,8R,9R,10R,13R,14R,17S,18R,19R)-17-hydrox...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase A assessed as release of phosphate from glucose-1-phosphate after 25 mins by microplate reader bas... | Eur J Med Chem 46: 2011-21 (2011) Article DOI: 10.1016/j.ejmech.2011.02.053 BindingDB Entry DOI: 10.7270/Q2DB826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||