

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM232964 US9353113, Ref. Ex. 2
SMILES: OC(=O)[C@H]1CC[C@H](CC(=O)N2CCc3c(C2)n(Cc2ccc(F)cc2F)c2ncccc32)CC1
InChI Key: InChIKey=PMDBHVLDYVKHEL-QAQDUYKDSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM232964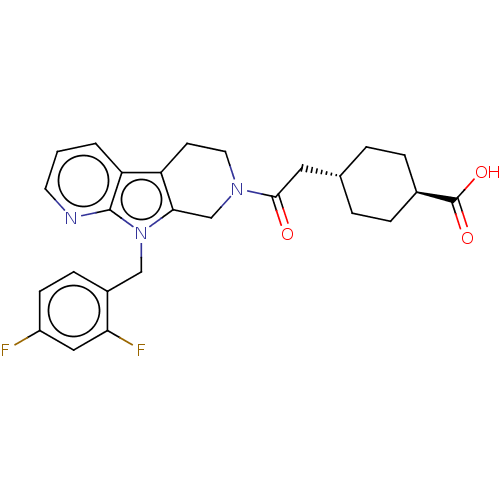 (US9353113, Ref. Ex. 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 9.0 | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description 10 μL of a test compound solution (10% dimethyl sulfoxide) at each concentration and 40 μL of a 5 μg/mL human ENPP2 solution (buffer A... | US Patent US9353113 (2016) BindingDB Entry DOI: 10.7270/Q20Z7250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM232964 (US9353113, Ref. Ex. 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of LysoPLD activity of ATX in human plasma assessed as reduction in choline release using LPC(16:0) as substrate incubated for 15 hrs | ACS Med Chem Lett 11: 1335-1341 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM232964 (US9353113, Ref. Ex. 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human ATX assessed as reduction in choline release using LPC(16:0) as substrate incubated for 15 hrs | ACS Med Chem Lett 11: 1335-1341 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||