

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CN(CCC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1)C(=O)c1c[nH]c(=O)o1
InChI Key: InChIKey=BYNGXNVERRUMKS-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM241121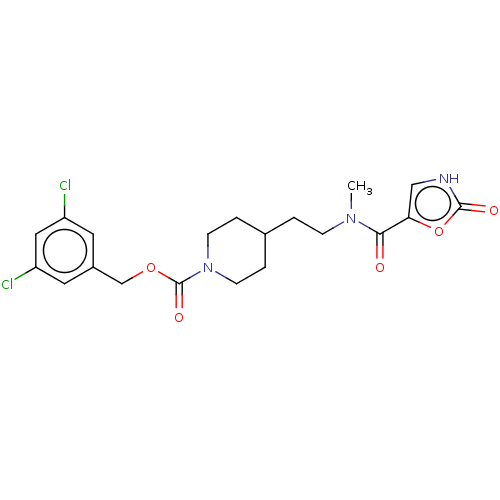 (US9409895, 32 | US9630945, 32) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample... | US Patent US9409895 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ataxin-2 (Homo sapiens (Human)) | BDBM241121 (US9409895, 32 | US9630945, 32) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description ATX activity was determined by measurement of released choline in reactions containing ATX (10 nM), choline oxidase (0.1 U/ml), HRP (100 U/ml), ample... | US Patent US9630945 (2017) BindingDB Entry DOI: 10.7270/Q25Q4Z5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM241121 (US9409895, 32 | US9630945, 32) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ATX (unknown origin) assessed as decrease in choline release | Bioorg Med Chem Lett 28: 2279-2284 (2018) Article DOI: 10.1016/j.bmcl.2018.05.030 BindingDB Entry DOI: 10.7270/Q2DB84DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM241121 (US9409895, 32 | US9630945, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells after 45 mins by scintillation counting method | Bioorg Med Chem Lett 28: 2279-2284 (2018) Article DOI: 10.1016/j.bmcl.2018.05.030 BindingDB Entry DOI: 10.7270/Q2DB84DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM241121 (US9409895, 32 | US9630945, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO-K1 cells at -90 mV holding potential by Qpatch clamp assay | Bioorg Med Chem Lett 28: 2279-2284 (2018) Article DOI: 10.1016/j.bmcl.2018.05.030 BindingDB Entry DOI: 10.7270/Q2DB84DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||