

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1cc2CCN(CCN(C)C)Cc2cc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1
InChI Key: InChIKey=RVSAJPCEEDMVAU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243429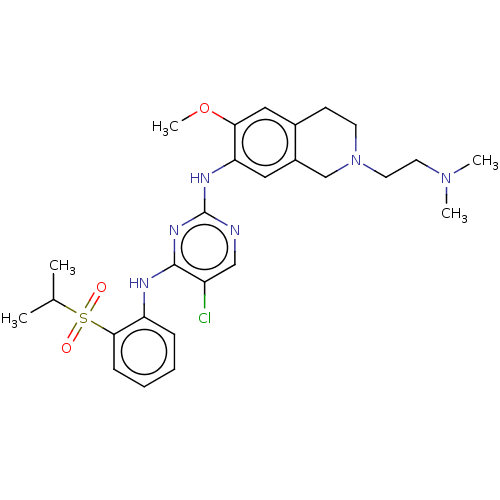 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(2-(2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243429 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(2-(2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of crizotinib-resistant ALK L1196M mutant (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescenc... | Citation and Details Article DOI: 10.1016/j.bmc.2015.12.004 BindingDB Entry DOI: 10.7270/Q269778Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243429 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(2-(2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ALK (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescence assay | Citation and Details Article DOI: 10.1016/j.bmc.2015.12.004 BindingDB Entry DOI: 10.7270/Q269778Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM243429 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(2-(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||