

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM246211 US10183932, Example 116::US9434690, 116::US9822102, Example 116
SMILES: COc1cn(C(CC2(C)CC2)C(=O)Nc2ccc(cc2)C(O)=O)c(=O)cc1-c1cc(Cl)ccc1C#N
InChI Key: InChIKey=DCFGHORVDDAJPY-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246211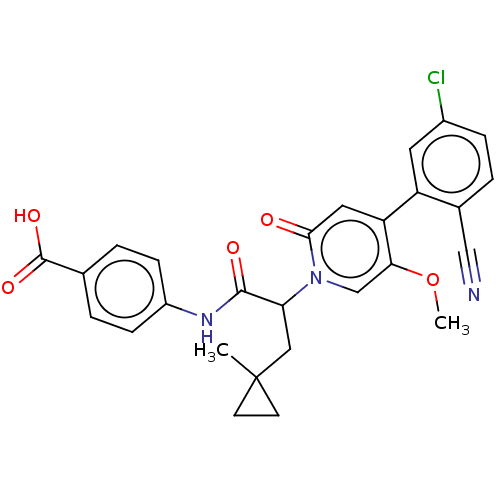 (US10183932, Example 116 | US9434690, 116 | US98221...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9434690 (2016) BindingDB Entry DOI: 10.7270/Q2K936FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM246211 (US10183932, Example 116 | US9434690, 116 | US98221...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9434690 (2016) BindingDB Entry DOI: 10.7270/Q2K936FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM246211 (US10183932, Example 116 | US9434690, 116 | US98221...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9822102 (2017) BindingDB Entry DOI: 10.7270/Q2FN18G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246211 (US10183932, Example 116 | US9434690, 116 | US98221...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of... | US Patent US10183932 (2019) BindingDB Entry DOI: 10.7270/Q23T9K99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246211 (US10183932, Example 116 | US9434690, 116 | US98221...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9822102 (2017) BindingDB Entry DOI: 10.7270/Q2FN18G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246211 (US10183932, Example 116 | US9434690, 116 | US98221...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of... | US Patent US10183932 (2019) BindingDB Entry DOI: 10.7270/Q23T9K99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||