

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM250042 US10005782, Compound 164::US9447106, 164::US9556188, Compound 164
SMILES: Cc1ccc(cc1)-c1nn2C(CCNc2c1C(N)=O)c1ccccc1NC(=O)C=C
InChI Key: InChIKey=BDFBZODVIHQKRH-UHFFFAOYSA-N
Data: 9 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM250042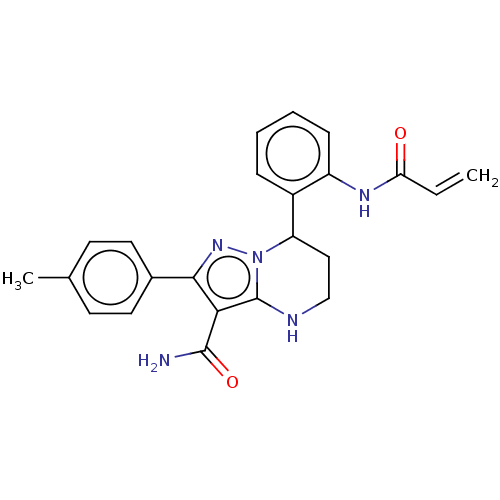 (US10005782, Compound 164 | US9447106, 164 | US9556...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | 25 |
BeiGene, Ltd. US Patent | Assay Description Compounds disclosed herein were tested for inhibition of Btk kinase activity in an assay based on time-resolved fluorescence resonance energy transfe... | US Patent US9447106 (2016) BindingDB Entry DOI: 10.7270/Q21Z439C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM250042 (US10005782, Compound 164 | US9447106, 164 | US9556...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | 25 |
BeiGene, Ltd. US Patent | Assay Description Compounds disclosed herein were tested for inhibition of Btk kinase activity in an assay based on time-resolved fluorescence resonance energy transfe... | US Patent US9556188 (2017) BindingDB Entry DOI: 10.7270/Q26Q2082 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM250042 (US10005782, Compound 164 | US9447106, 164 | US9556...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Compounds disclosed herein were tested for inhibition of Btk kinase activity in an assay based on time-resolved fluorescence resonance energy transfe... | Bioorg Med Chem 17: 7755-68 (2009) BindingDB Entry DOI: 10.7270/Q29889BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM250042 (US10005782, Compound 164 | US9447106, 164 | US9556...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) preincubated for 1 hr followed by Biotin-AVLESEEELYSSARQ-NH2 substrate addition in presence of ATP and measured a... | J Med Chem 62: 7923-7940 (2019) Article DOI: 10.1021/acs.jmedchem.9b00687 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM250042 (US10005782, Compound 164 | US9447106, 164 | US9556...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) preincubated for 1 hr followed by Biotin-AVLESEEELYSSARQ-NH2 substrate addition in presence of ATP and measured a... | J Med Chem 62: 7923-7940 (2019) Article DOI: 10.1021/acs.jmedchem.9b00687 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM250042 (US10005782, Compound 164 | US9447106, 164 | US9556...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos cells assessed as reduction in BTK phosphorylation at Tyr223 residue preincubated for 1 hr followed by pervanadate o... | J Med Chem 62: 7923-7940 (2019) Article DOI: 10.1021/acs.jmedchem.9b00687 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM250042 (US10005782, Compound 164 | US9447106, 164 | US9556...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged recombinant human BTK (393 to 659 residues) expressed in baculovirus infected Sf9 cells preincubated for 1 hr fol... | J Med Chem 62: 7923-7940 (2019) Article DOI: 10.1021/acs.jmedchem.9b00687 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM250042 (US10005782, Compound 164 | US9447106, 164 | US9556...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged recombinant human BTK (393 to 659 residues) expressed in baculovirus infected Sf9 cells preincubated for 1 hr fol... | J Med Chem 62: 7923-7940 (2019) Article DOI: 10.1021/acs.jmedchem.9b00687 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM250042 (US10005782, Compound 164 | US9447106, 164 | US9556...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos cells assessed as reduction in BTK phosphorylation at Tyr223 residue preincubated for 1 hr followed by pervanadate o... | J Med Chem 62: 7923-7940 (2019) Article DOI: 10.1021/acs.jmedchem.9b00687 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||