

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM26229 2-Aminopyrimidine analog., 4::4-[2-amino-6-(4-methylpiperazin-1-yl)pyrimidin-4-yl]benzonitrile::JMC516547 Compound 2
SMILES: CN1CCN(CC1)c1cc(nc(N)n1)-c1ccc(cc1)C#N
InChI Key: InChIKey=QFKQUMBUFVKQBH-UHFFFAOYSA-N
Data: 6 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM26229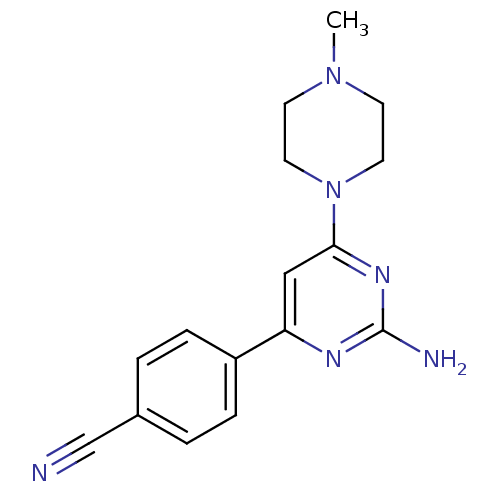 (2-Aminopyrimidine analog., 4 | 4-[2-amino-6-(4-met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 35 | -10.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6571-80 (2008) Article DOI: 10.1021/jm8005959 BindingDB Entry DOI: 10.7270/Q24J0CD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM26229 (2-Aminopyrimidine analog., 4 | 4-[2-amino-6-(4-met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 35 | -10.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM26229 (2-Aminopyrimidine analog., 4 | 4-[2-amino-6-(4-met...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 245 | -9.01 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6571-80 (2008) Article DOI: 10.1021/jm8005959 BindingDB Entry DOI: 10.7270/Q24J0CD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 Receptor (Rattus norvegicus (rat)) | BDBM26229 (2-Aminopyrimidine analog., 4 | 4-[2-amino-6-(4-met...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 275 | -8.94 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6571-80 (2008) Article DOI: 10.1021/jm8005959 BindingDB Entry DOI: 10.7270/Q24J0CD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 Receptor (Rattus norvegicus (rat)) | BDBM26229 (2-Aminopyrimidine analog., 4 | 4-[2-amino-6-(4-met...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 275 | -8.94 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM26229 (2-Aminopyrimidine analog., 4 | 4-[2-amino-6-(4-met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.29E+3 | -8.03 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6571-80 (2008) Article DOI: 10.1021/jm8005959 BindingDB Entry DOI: 10.7270/Q24J0CD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||