

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM291683 1-(4-(((6-amino-5-(4-(pyridin-4-yloxy)phenyl)pyrimidin-4-yl)amino)methyl)piperidin-1-yl)prop-2-en-1-one ::US10413562, Compound A97::US9580449, Example A97
SMILES: Nc1ncnc(NCC2CCN(CC2)C(=O)C=C)c1-c1ccc(Oc2ccncc2)cc1
InChI Key: InChIKey=YMJCJOSTNRHERU-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291683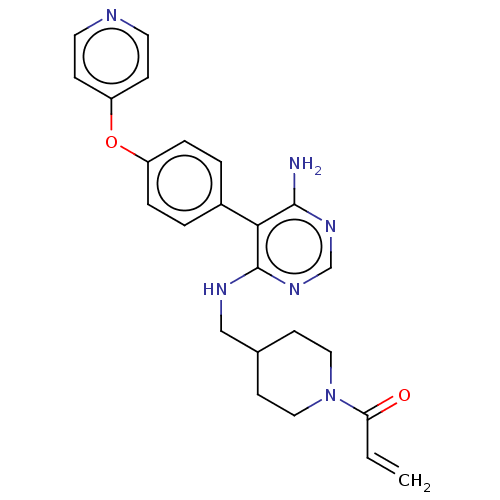 (1-(4-(((6-amino-5-(4-(pyridin-4-yloxy)phenyl)pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 27 |
Merck Patent GmbH US Patent | Assay Description The following describes a microfluidic, off-chip mobility shift kinase assay used to measure inherent potency of compounds against BTK enzyme. Compou... | US Patent US9580449 (2017) BindingDB Entry DOI: 10.7270/Q28K7C4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291683 (1-(4-(((6-amino-5-(4-(pyridin-4-yloxy)phenyl)pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291683 (1-(4-(((6-amino-5-(4-(pyridin-4-yloxy)phenyl)pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description 2.5× stocks of full-length human BTK (08-080) from CarnaBio USA, Inc., Natick, Mass., 1.6×ATP and appropriate kinKDR peptide substrate (FITC-AHA-EEPL... | US Patent US10413562 (2019) BindingDB Entry DOI: 10.7270/Q27M0B95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||