

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Clc1cnc2nc(N3CCNCC3)n3nc(nc3c2c1)C1CC1
InChI Key: InChIKey=DILJIYOWMILJQS-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM294381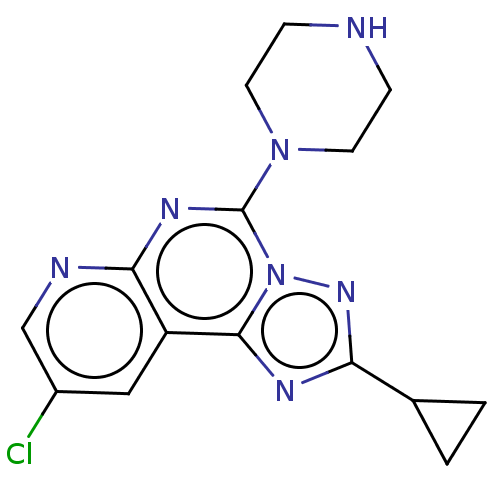 (US9586959, Compound 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | 7.4 | 27 |
C&C RESEARCH LABORATORIES US Patent | Assay Description Each compound of the present invention prepared in the examples was prepared in DMSO into concentrations of 0.02, 0.06, 0.3 and 2 mM. 10 uL of the pr... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294381 (US9586959, Compound 39) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||