

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM296301 US10112937, Example 404
SMILES: CCn1ccc(n1)-n1nnc2CN([C@@H](C)Cc12)C(=O)c1cccc(c1Cl)C(F)(F)F
InChI Key: InChIKey=JKXUCUMFOJGLGA-NSHDSACASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM296301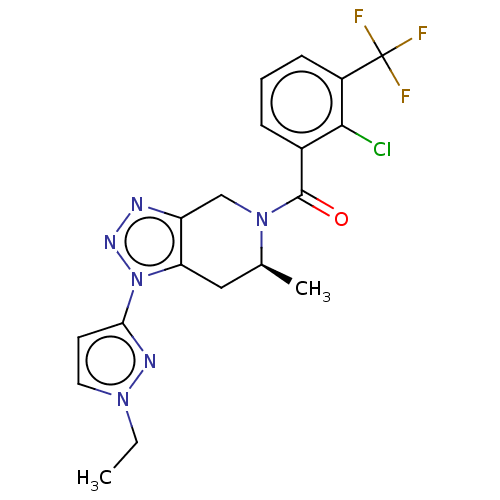 (US10112937, Example 404) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM296301 (US10112937, Example 404) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description 1321N1 cells expressing the recombinant human or rat P2X7 channel was cultured in HyQ DME/(HyClone/Dulbecco's Modified Eagle Medium) high glucose... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM296301 (US10112937, Example 404) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide or diclofenac as substrates | J Med Chem 61: 207-223 (2018) Article DOI: 10.1021/acs.jmedchem.7b01279 BindingDB Entry DOI: 10.7270/Q26T0Q2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM296301 (US10112937, Example 404) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant P2X7 expressed in 1321N1 cells assessed as inhibition of BzATP-induced calcium mobilization after 30 mins by... | J Med Chem 61: 207-223 (2018) Article DOI: 10.1021/acs.jmedchem.7b01279 BindingDB Entry DOI: 10.7270/Q26T0Q2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM296301 (US10112937, Example 404) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using S-Mephenytoin as substrate | J Med Chem 61: 207-223 (2018) Article DOI: 10.1021/acs.jmedchem.7b01279 BindingDB Entry DOI: 10.7270/Q26T0Q2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM296301 (US10112937, Example 404) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description 1321N1 cells expressing the recombinant human or rat P2X7 channel was cultured in HyQ DME/(HyClone/Dulbecco's Modified Eagle Medium) high glucose... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM296301 (US10112937, Example 404) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant P2X7 expressed in 1321N1 cells assessed as inhibition of BzATP-induced calcium mobilization after 30 mins by c... | J Med Chem 61: 207-223 (2018) Article DOI: 10.1021/acs.jmedchem.7b01279 BindingDB Entry DOI: 10.7270/Q26T0Q2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||