

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM298439 4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsulfonamido)phenyl)ureido)naphthalen-1-yl)oxy)pyridin-2-yl)amino)-2,6-dimethoxy-N-(2-(4-methylpiperazin-1-yl)ethyl)benzamide::US10125100, Example 22::US10392346, Example 22::US10941115, Example 22::US9751837, Example 22
SMILES: COc1cc(Nc2cc(Oc3ccc(NC(=O)Nc4cc(cc(NS(C)(=O)=O)c4OC)C(C)(C)C)c4ccccc34)ccn2)cc(OC)c1C(=O)NCCN1CCN(C)CC1
InChI Key: InChIKey=KLCRZBNGDBKHFA-UHFFFAOYSA-N
Data: 13 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM298439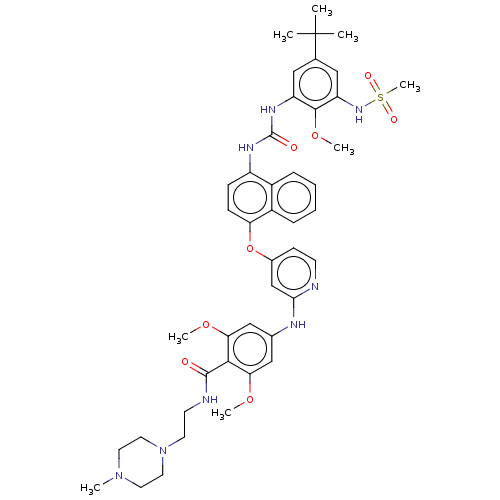 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen) are evaluated indirectly by determining the level... | US Patent US10125100 (2018) BindingDB Entry DOI: 10.7270/Q2XW4MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298439 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US10125100 (2018) BindingDB Entry DOI: 10.7270/Q2XW4MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM298439 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US10125100 (2018) BindingDB Entry DOI: 10.7270/Q2XW4MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM298439 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description ompounds of the invention were tested for inhibition of the human ether a go-go (hERG) channel using IonWorks patch clamp electrophysiology at Essen ... | US Patent US10125100 (2018) BindingDB Entry DOI: 10.7270/Q2XW4MV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM298439 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9751837 (2017) BindingDB Entry DOI: 10.7270/Q2028TP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298439 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9751837 (2017) BindingDB Entry DOI: 10.7270/Q2028TP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM298439 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxular Acquisitions Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10941115 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM298439 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM298439 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM298439 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| p38 MAP kinase alpha/beta (Homo sapiens (Human)) | BDBM298439 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxular Acquisitions Limited US Patent | Assay Description p38 MAPKα Method 2: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen) are evaluated indirectly... | US Patent US10941115 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein cereblon/Tyrosine-protein kinase SRC (Homo sapiens (Human)) | BDBM298439 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxular Acquisitions Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10941115 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM298439 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description Method 1The inhibitory activities of compounds of the invention against the GSK 3α enzyme isoform (Invitrogen), are evaluated by determining the... | US Patent US9751837 (2017) BindingDB Entry DOI: 10.7270/Q2028TP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||