

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM30331 Naphthoic acid-based analog, 1d
SMILES: CC(C)c1onc(c1COc1ccc(cc1)-c1ccc2ccc(cc2c1)C(O)=O)-c1c(Cl)cccc1Cl
InChI Key: InChIKey=GSTNOFVRIRGPDE-UHFFFAOYSA-N
Data: 2 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bile acid receptor (Homo sapiens (Human)) | BDBM30331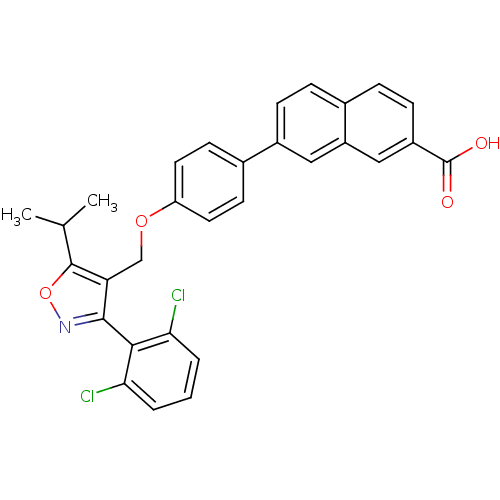 (Naphthoic acid-based analog, 1d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
GSK | Assay Description The cell-based assay measures the ligand-mediated luminescense resulting from FXR-induced transcription of a luciferase reporter. FXR and the lucifer... | Bioorg Med Chem Lett 18: 4339-43 (2008) Article DOI: 10.1016/j.bmcl.2008.06.073 BindingDB Entry DOI: 10.7270/Q2KK994P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM30331 (Naphthoic acid-based analog, 1d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 420 | n/a | n/a | 7.5 | 22 |
GSK | Assay Description The assay measures ligand-mediated interaction of the SRC-1 peptide with the FXR ligand binding domain, using biotinylated FXR LBD coupled to allophy... | Bioorg Med Chem Lett 18: 4339-43 (2008) Article DOI: 10.1016/j.bmcl.2008.06.073 BindingDB Entry DOI: 10.7270/Q2KK994P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||