

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1ccc2N=C(NS(=O)(=O)c2c1)c1c(O)c2cccn2n(CCC(C)C)c1=O
InChI Key: InChIKey=YJZNZDXWNSYVDU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM30409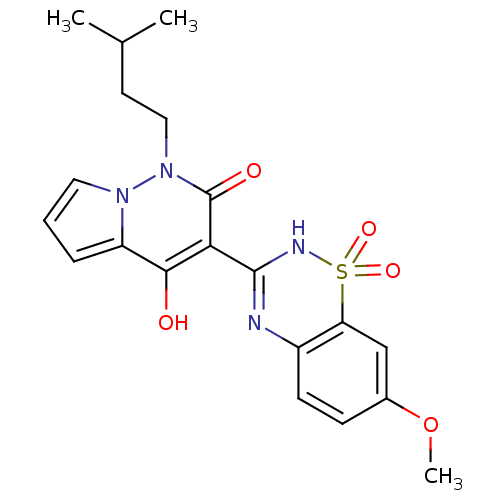 (pyrrolo[1,2-b]pyridazin-2-one analog., 3b) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | 1.70E+4 | n/a | n/a | 7.5 | 28 |
Anadys Pharmaceuticals | Assay Description Assays were performed in a 96-well streptavidin-coated Flash-Plate using enzyme, RNA substrate, and [alpha-33P]GTP/GTP with inhibitor concentration v... | Bioorg Med Chem Lett 18: 3616-21 (2008) Article DOI: 10.1016/j.bmcl.2008.04.066 BindingDB Entry DOI: 10.7270/Q26D5R9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||